Advanced Microscopy and Optical Tweezing
The group has led the way in developing adaptive optics in optical microscopy in particular in non-linear microscopy and light sheet (SPIM) imaging. In the case of SPIM the work advanced to using a heart synchronisation process to “freeze” a beating heart so that 3D optical images could be generated at high speed throughout the heart cycle. CfAI also introduced the use of an extra beam in a SPIM system, entering through the imaging arm, to ablate individual cells within the fish that had been genetically encoded with KillerRED. This work has now advanced to use optical tweezers to examine what happens as neutrophils “digest” unwanted bacteria and other disease items.
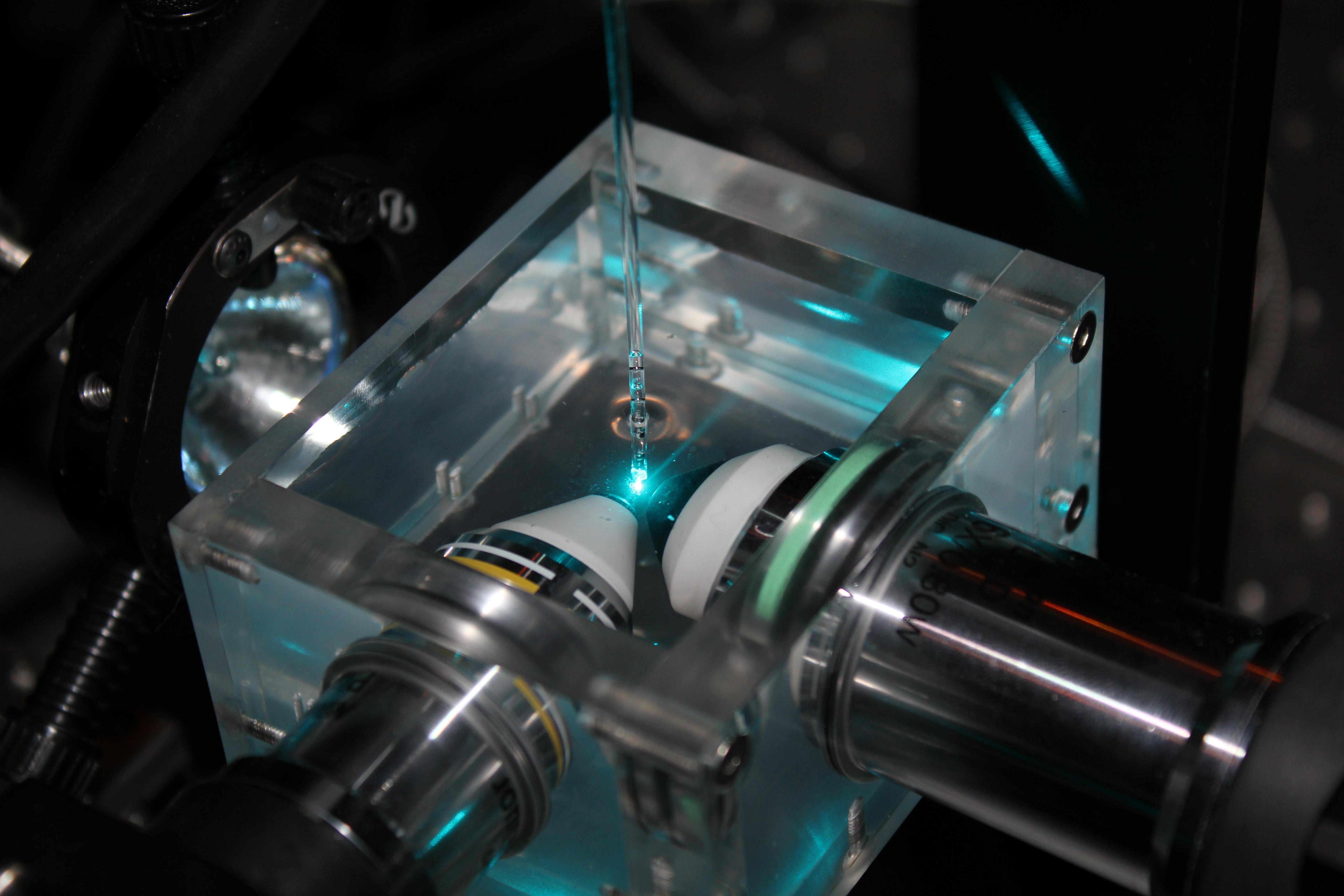
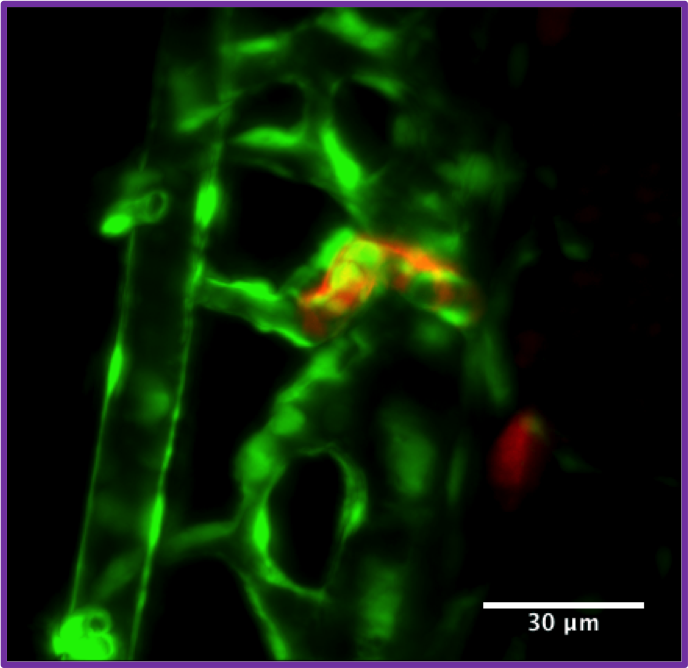
SPIM image of a Zebrafish developing kidney (red) with developing vasculature in green.


/prod01/prodbucket01/media/durham-university/departments-/physics/cfai/CfAI-Webpage-Banner-smaller.jpg)