Staff profile
Overview
https://apps.dur.ac.uk/biography/image/1327
Dr Matthew Kitching
Associate Professor

| Affiliation | Telephone |
|---|---|
| Associate Professor in the Department of Chemistry | +44 (0) 191 33 42127 |
| Fellow of the Wolfson Research Institute for Health and Wellbeing |
Biography
Kitching Group Summary
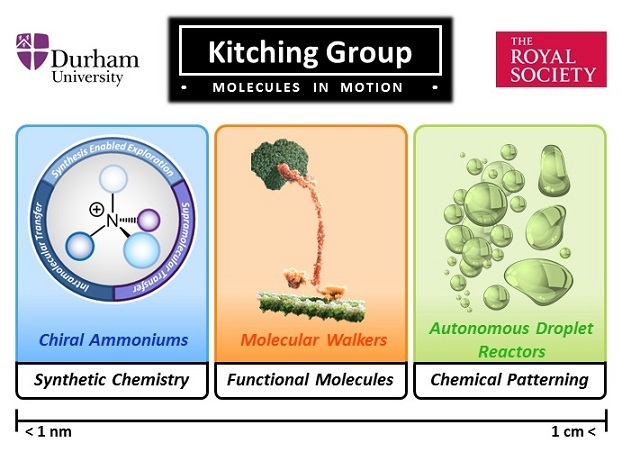
Image of walker adapted from John Lieber's image at https://www.artofthecell.com/
Research interests
- Synthetic Chemistry
- Supramolecular Chemistry & Functional Molecules
- Chemical Patterning, Soft Materials, and Interfaces
Publications
Journal Article
- Conglomerate Crystallization in the Cambridge Structural Database (2020–2021)Walsh, M. P., Barclay, J. A., Begg, C. S., Xuan, J., & Kitching, M. O. (2023). Conglomerate Crystallization in the Cambridge Structural Database (2020–2021). Crystal Growth & Design, 23(4), 2837-2837. https://doi.org/10.1021/acs.cgd.3c00019
- Supramolecular Recognition of Quaternary Phosphonium CationsWalsh, M. P., & Kitching, M. O. (2022). Supramolecular Recognition of Quaternary Phosphonium Cations. Crystal Growth & Design, 22(1), 251-258. https://doi.org/10.1021/acs.cgd.1c00887
- Identifying a Hidden Conglomerate Chiral Pool in the CSDWalsh, M. P., Barclay, J. A., Begg, C. S., Xuan, J., Johnson, N. T., Cole, J. C., & Kitching, M. O. (2022). Identifying a Hidden Conglomerate Chiral Pool in the CSD. JACS Au, 2(10), 2235-2250. https://doi.org/10.1021/jacsau.2c00394
- Enantioselective synthesis of ammonium cationsWalsh, M. P., Phelps, J. M., Lennon, M. E., Yufit, D. S., & Kitching, M. O. (2021). Enantioselective synthesis of ammonium cations. Nature, 597(7874), 70-76. https://doi.org/10.1038/s41586-021-03735-5
- Regioselective Functionalization of 7‐Azaindole by Controlled Annular Isomerism: The Directed Metalation‐Group DanceSnieckus, V., Patel, J., Kitching, M., Dalziel, M., Cosman, J., & Kaye, M. (2019). Regioselective Functionalization of 7‐Azaindole by Controlled Annular Isomerism: The Directed Metalation‐Group Dance. Angewandte Chemie, 131(22), 7391-7395. https://doi.org/10.1002/ange.201901724
- Directed Remote Lateral Metalation: Highly Substituted 2-Naphthols and BINOLs by in situ Directing Group GenerationSnieckus, V., Kitching, M., Laars, M., Board, J., Patel, J., & Gan, W. (2018). Directed Remote Lateral Metalation: Highly Substituted 2-Naphthols and BINOLs by in situ Directing Group Generation. Angewandte Chemie, 130(30), 9569-9573. https://doi.org/10.1002/ange.201805203
- Directed Remote Lateral Metalation: Highly Substituted 2-Naphthols and BINOLs by In Situ Generation of a Directing GroupPatel, J. J., Laars, M., Gan, W., Board, J., Kitching, M. O., & Snieckus, V. (2018). Directed Remote Lateral Metalation: Highly Substituted 2-Naphthols and BINOLs by In Situ Generation of a Directing Group. Angewandte Chemie International Edition, 57(30), 9425-9429. https://doi.org/10.1002/anie.201805203
- Flow-Assisted Synthesis: A Key Fragment of SR 142948AKitching, M. O., Dixon, O. E., Baumann, M., & Baxendale, I. R. (2017). Flow-Assisted Synthesis: A Key Fragment of SR 142948A. European Journal of Organic Chemistry, 2017(44), 6540-6553. https://doi.org/10.1002/ejoc.201700904
- Sequence-Specific β-Peptide Synthesis by a Rotaxane-Based Molecular MachineDe Bo, G., Gall, M. A., Kitching, M. O., Kuschel, S., Leigh, D. A., Tetlow, D. J., & Ward, J. W. (2017). Sequence-Specific β-Peptide Synthesis by a Rotaxane-Based Molecular Machine. Journal of the American Chemical Society, 139(31), 10875-10879. https://doi.org/10.1021/jacs.7b05850
- Tying a Molecular Overhand Knot of Single Handedness and Asymmetric Catalysis with the Corresponding Pseudo-D-3-Symmetric Trefoil KnotGil-Ramirez, G., Hoekman, S., Kitching, M. O., Leigh, D. A., Vitorica-Yrezabal, I. J., & Zhang, G. (2016). Tying a Molecular Overhand Knot of Single Handedness and Asymmetric Catalysis with the Corresponding Pseudo-D-3-Symmetric Trefoil Knot. Journal of the American Chemical Society, 138(40), 13159-13162. https://doi.org/10.1021/jacs.6b08421
- Metal-Free Synthesis of Dibenzoxazepinones via a One-Pot SNAr and Smiles Rearrangement Process: Orthogonality with Copper-Catalyzed CyclizationsHurst, T. E., Kitching, M. O., da Frota, L. C., Guimaraes, K. G., Dalziel, M. E., & Snieckus, V. (2015). Metal-Free Synthesis of Dibenzoxazepinones via a One-Pot SNAr and Smiles Rearrangement Process: Orthogonality with Copper-Catalyzed Cyclizations. Synlett: Accounts and Rapid Communications in Synthetic Organic Chemistry, 26(11), 1455-1460. https://doi.org/10.1055/s-0034-1378839
- Goldberg Active Template Synthesis of a [2]Rotaxane Ligand for Asymmetric Transition-Metal CatalysisHoekman, S., Kitching, M. O., Leigh, D. A., Papmeyer, M., & Roke, D. (2015). Goldberg Active Template Synthesis of a [2]Rotaxane Ligand for Asymmetric Transition-Metal Catalysis. Journal of the American Chemical Society, 137(24), 7656-7659. https://doi.org/10.1021/jacs.5b04726
- The synthesis of neurotensin antagonist SR 48692 for prostate cancer researchBaxendale, I., Cheung, S., Kitching, M., Ley, S., & Shearman, J. (2013). The synthesis of neurotensin antagonist SR 48692 for prostate cancer research. Bioorganic and Medicinal Chemistry, 21(14), 4378-4387. https://doi.org/10.1016/j.bmc.2013.04.075
- A Machine-Assisted Flow Synthesis of SR48692: A Probe for the Investigation of Neurotensin Receptor-1Battilocchio, C., Deadman, B. J., Nikbin, N., Kitching, M. O., Baxendale, I. R., & Ley, S. V. (2013). A Machine-Assisted Flow Synthesis of SR48692: A Probe for the Investigation of Neurotensin Receptor-1. Chemistry - A European Journal, 19(24), 7917-7930. https://doi.org/10.1002/chem.201300696
- A Flow-Based Synthesis of 2-Aminoadamantane-2-carboxylic AcidBattilocchio, C., Baxendale, I. R., Biava, M., Kitching, M. O., & Ley, S. V. (2012). A Flow-Based Synthesis of 2-Aminoadamantane-2-carboxylic Acid. Organic Process Research and Development, 16(5), 798-810. https://doi.org/10.1021/op300084z
- Scale-Up of Flow-Assisted Synthesis of C-2-Symmetric Chiral PyBox LigandsBattilocchio, C., Baumann, M., Baxendale, I. R., Biava, M., Kitching, M. O., Ley, S. V., Martin, R. E., Ohnmacht, S. A., & Tappin, N. D. (2012). Scale-Up of Flow-Assisted Synthesis of C-2-Symmetric Chiral PyBox Ligands. Synthesis : Journal of Synthetic Organic Chemistry., 44(4), 635-647. https://doi.org/10.1055/s-0031-1289676
- Organic chemistry - Directors extend their reachKitching, M. O., & Snieckus, V. (2012). Organic chemistry - Directors extend their reach. Nature, 486(7404), 478-479. https://doi.org/10.1038/486478a
- Copper-Catalyzed Cross-Coupling Interrupted by an Opportunistic Smiles Rearrangement: An Efficient Domino Approach to DibenzoxazepinonesKitching, M. O., Hurst, T. E., & Snieckus, V. (2012). Copper-Catalyzed Cross-Coupling Interrupted by an Opportunistic Smiles Rearrangement: An Efficient Domino Approach to Dibenzoxazepinones. Angewandte Chemie International Edition, 51(12), 2925-2929. https://doi.org/10.1002/anie.201106786
- Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel PrizeSeechurn, C. C. J., Kitching, M. O., Colacot, T. J., & Snieckus, V. (2012). Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angewandte Chemie International Edition, 51(21), 5062-5085. https://doi.org/10.1002/anie.201107017
- Enzymatic Oxidative Cyclisation Reactions Leading to DibenzoazocanesTozzi, F., Ley, S. V., Kitching, M. O., & Baxendale, I. R. (2010). Enzymatic Oxidative Cyclisation Reactions Leading to Dibenzoazocanes. Synlett: Accounts and Rapid Communications in Synthetic Organic Chemistry, 2010(13), 1919-1922. https://doi.org/10.1055/s-0030-1258486
- Cancer, Chemistry, and the Cell: Molecules that Interact with the Neurotensin ReceptorsMyers, R. M., Shearman, J. W., Kitching, M. O., Ramos-Montoya, A., Neal, D. E., & Ley, S. V. (2009). Cancer, Chemistry, and the Cell: Molecules that Interact with the Neurotensin Receptors. ACS Chemical Biology, 4(7), 503-525. https://doi.org/10.1021/cb900038e
- (S)-5-PYRROLIDIN-2-YL-1H-TETRAZOLEAureggi, V., Franckevičius, V., Kitching, M., Ley, S., Longbottom, D., Oelke, A., & Sedelmeier, G. (2008). (S)-5-PYRROLIDIN-2-YL-1H-TETRAZOLE. Organic Syntheses, 85(72). https://doi.org/10.15227/orgsyn.085.0072
- New opportunities for advanced organic synthesis-flow-based chemical processingBaumann, M., Baxendale, I. R., Hayward, J. J., Hopkin, M. D., Jin, J., Kitching, M. O., Lanners, S., Ley, S. V., Nikbin-Roudsari, N., Smith, C. D., Smith, C. J., & Tamborini, L. (2008). New opportunities for advanced organic synthesis-flow-based chemical processing. Journal Of Labelled Compounds & Radiopharmaceuticals, 51(5-6), 252-253.
Supervision students
Alex Lee
Corey Stewart
Research Postgraduate
Diego Alvarez Bustos
Research Postgraduate
Elle Fiandra
Research Postgraduate
Haoyang Wang
Jamie Hunter
Research Postgraduate
Riccardo Parolin
Demonstrator (Ptt)

