Staff profile
Professor Andrew Whiting
Emeritus Professor

| Affiliation | Telephone |
|---|---|
| Emeritus Professor in the Department of Chemistry |
Biography
PhD (1981-’84): University of Newcastle upon Tyne, working on beta-lactam chemistry. Post-doctoral research (1984-’86): Boston College, MA, USA, working on natural product synthesis and asymmetric synthesis and developing chiral Diels-Alder Lewis-acid catalysts. Industrial experience (1986-’88): Ciba-Geigy plc., Central Research UK, working on varied topics, including developing new methodology for the preparation of novel amino-acid analogues and asymmetric synthesis. Academic experience: Chemistry Department, UMIST (1989-2001), moved to Readership at Durham (2001) and promoted to Professor in 2009.
Current Research Interests
The major activity is increasingly related to innovation, working with two spinout companies setup through research carried in our group, i.e. LightOx Ltd. (lightox.co.uk) and more recently, Nevrargenics Ltd. (nevrargenics.com). These two companies are driving forward novel drugs in the areas of phototherapeutic therapy and neurodegenerative disease treatment, respectively.
Our research themes revolve more around chemical biology more recently, but our background efforts have been in organometallic chemistry, catalysis, asymmetric and stereoselective synthesis, particularly looking a new, cleaner, greener and water tolerant catalytic processes. These areas are exemplified below by some recent published papers:
Bifunctional catalysis: synthesis and applications of aminoboronic acids
We are interested in developing new methods for the synthesis of organoboron compounds for a number of reasons (see below) and
one major ongoing project in this area involves the design, synthesis and
development of new bifunctional catalysts based on amino-boronic acids. For example, homoboroproline is an excellent enamine-based organic/bifunctional catalyst especially when the boron Lewis acidity is tuned in situ by esterification, as shown in the reaction below, and we have reported a detailed study of how this catalyst system works, as well as making both enantiomers available and producing effectively 98% e.e.1
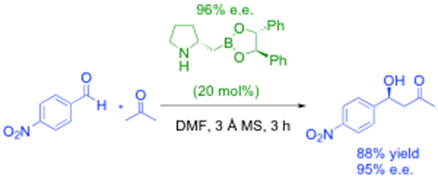
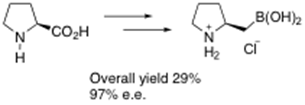
We have also recently developed an efficient highly enantioselective synthesis of the enantiomeric catalyst using an Sn2-like borylation route from natural proline, i.e.:2
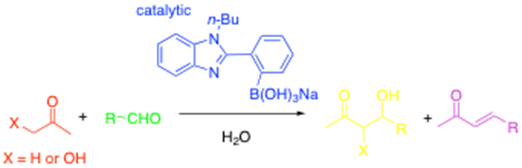
Bifunctional catalysts can also be used to generate boronate enolates in situ and in water! In this case, we found that a phenylbenzimidazole boronate 'ate'-complex could catalyse to aldol reaction as follows:3
Direct amide formation
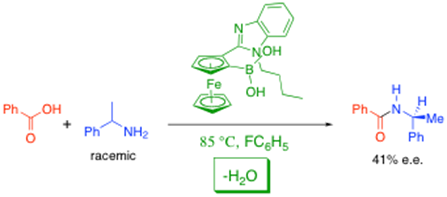
Despite what we are told at undergraduate level, direct amide formation from amines and carboxylic acids works, and without the need to use atom uneconomic methods. We have been working on the development of catalysts to accomplish this reaction under increasingly lower temperature conditions, especially by using organoboron bifunctional catalysts and other activated aryl boronic acids.4 We have even reported the first example an asymmetric process involving a chiral boronic acid catalyst which can achieve kinetic resolution of a racemic amine through direct amide formation, as shown below.5
We have also delved into the heavily misunderstood uncatalysed reaction which is much more facile than many working in the area realise! We have proposed, backed up by theoretical calculations, that hydrogen-bonded dimeric carboxylic acid complexes could well be involved in such reactions, as outlined below.6a
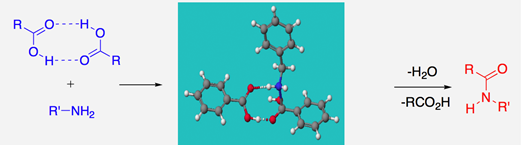
This mechanism contrasts with recent work that has uncovered the hitherto hidden workings of boron-based amidation catalysis.6b In fact, we now understand that amidation catalysis by boron requires two boron atoms, acting cooperatively, particularly through B-X-B bridged systems, where X = O or N, with the two borons acting as bridges to doubly activate carboxylic groups; mechanistic proposals which have been reinforced by extensive DFT calculations.
Nitroso- and imino-dienophile based formal aza-Diels-Alder reactions: novel approaches to nitrogen heterocycles
Our interest in developing novel routes to nitrogen heterocycles using clean catalytic methods goes back many years, particularly involving imino dienophiles, for example, using zinc(II) binol complexes as shown below,7 and has recently been extended to look at a range imine-mediated formal cycloaddition reactions, to access a number of different hydropyridine analogues.7
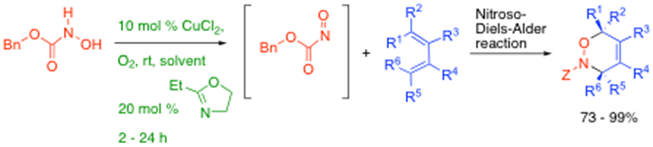
In addition, we have been developing novel copper-based air oxidative formation of acyl nitroso species from hydroxamic acids, which are then efficiently trapped out as Diels-Alder adducts, such as shown below, and in conjunction with Dr Bertrand Carboni (Rennes University) we have aalso reported the reaction of nitroso compounds with borodienes to access pyrroles, amongst other things.8
Borylation of unsaturated imines
We are interested in developing new, catalytic, asymmetric routes to gamma-amino alcohols and beta-amino acids etc, and together with Prof Elena Fernández' group at the Universitat Rovira i Virgili in Tarragona, a rapid and efficient entry to such systems is being developed using borylation of unsaturated imines, i.e. as summarised below, and has been extended to synthesis of antidepressants, and even applied to highly reactive unsaturated aldehyde analogues.9
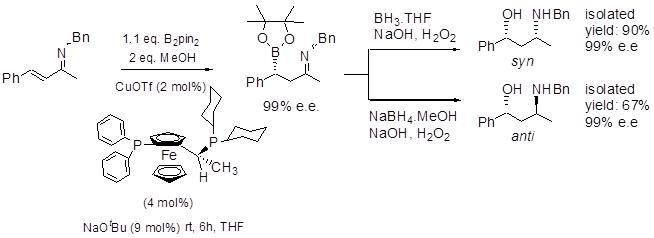
Stereocontrolled polyene natural product synthesis
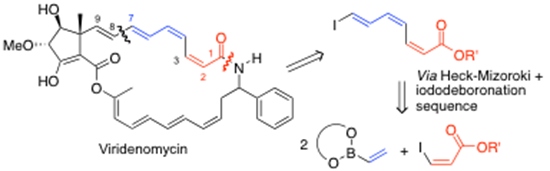
We have pioneered the use of vinylboronate esters which undergo Heck-Mizoroki coupling to derive dienyl and polyenyl-boronates systems. This is a powerful technology when coupled with highly efficient iodo-deboronation methods, which can be carried out with either inversion or retention of stereochemistry. The additional use of Suzuki-Miyaura cross-coupling completes the technology to access highly sensitive polyene systems, particularly with cis-alkene geometries present and we are currently part-way through the total synthesis of the anti-leukemic agent viridenomycin using this technology, i.e. as outlined here:10
Design and synthesis of organic compounds for controlled cellular development
We collaborate widely with research groups in Biology (Dr Carrie Ambler & Dr Paul Chazot), Psychology (Dr Alex easton and Dr David Sanderson) and Physics (Prof John Girkin), here in Chemistry (Dr Ehmke Pohl), the Northern Centre for Cancer Research, Newcastle (Dr Chris Redfern), High Force Research (Dr Roy Valentine), and with the University of
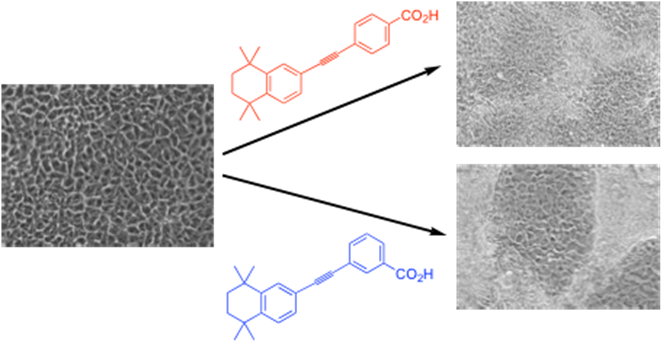
Aberdeen (Dr Iain Greig and Prof Peter McCaffery). Our role has been the design, modelling and synthesis of synthetic retinoids, i.e. analogues of retinoic acids, including all-trans-retinoic acid, ATRA). These types of systems act as molecular triggers causing changes in cellular development processes. For example, we have been developing light and heat stable analogues (such as EC23 and EC1911) of ATRA and its isomers which are still capable of inducing cell differentiation in stem cell systems deriving neural cells in the case of EC23. Recently, this owrk has led to the identification of synthetic retinoids with the potential to treat neurodegenerative diseases, which we are taking forwards through the spinout company Nevrargenics (https://nevrargenics.com/).
In addition, we have been developing novel copper-based air oxidative formation of acyl nitroso species from hydroxamic acids, which are then efficiently trapped out as Diels-Alder adducts, such as shown below, and in conjunction with Dr Bertrand Carboni (Rennes University) we have also reported the reaction of nitroso compounds with borodienes to access
pyrroles, amongst other things.8
In addition, we have now created fluorescent variants, which we are using for bioimaging purposes12 and other medical applications, including cell killing using systems specially designed to be particularly fluorescent. A spinout company, LightOx Ltd. has also been setup to commercialise this work involving the sale of fluorescent imaging probes and the development novel phototherapeutic drugs.
New materials directed synthesis: novel, environmentally benign approaches to crosslinked polymers
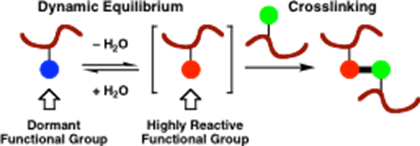
We have an ongoing project involving patented technology developed jointly with the Materials Science department at Manchester, which has involved the design and synthesis of bifunctional crosslinking polymer monomers for application in water-borne latex coating systems. For example, HydroxyEthylSulfonylStyrene (HESS)13 is a reactive monomer species which was developed by us to demonstrate the concept in a new entirely-water based crosslinking system. This type of crosslinking system only forms crosslinks upon coating and evaporation of water
from the emulsion system due to the formation of reactive vinylsulfones which under facile addition reactions with, for example, hydroxyl groups, i.e. as schematically shown below. This area is being further developed currently for decorative coatings in collaboration with AkzoNobel.
Acknowledgements
We would like to thank the following for support over the years (not all of which still exist!): EPSRC, BBSRC, MRC, Royal Society, RSC, GSK, Schering-Plough, Syngenta, Pfizer, High Force Research, Merck, AkzoNobel, Knoll Pharmaceuticals, Johnson-Matthey, Holliday Dyes &
Chemicals, ICI Surfactants, Chiroscience, Schlumberger Cambridge Research and Ciba-Geigy.
References
1. a) I. Georgiou, A. Whiting, Org. Biomol. Chem., 2012, 10, 2422-2430, DOI:10.1039/C2OB06872A; b) A. S. Batsanov, I. Georgiou, P. R. Girling, L. Pommier, H. C. Shen and A. Whiting, Asian J. Org. Chem., 2014, 3, 470-479, DOI: 10.1002/ajoc.201300127
2. I. Georgiou, A. Whiting, Eur. J. Org. Chem., 2012, 4110–4113, DOI: 10.1002/ejoc.201200652
3. K. Aelvoet, A. S. Batsanov, A. J. Blatch, L. G. F. Patrick, C. A. Smethurst, A. Whiting, Angew. Chem., 2008, 47, 768-770, DOI: 10.1002/anie.200705643
4. a) K. Arnold, A. S. Batsanov, B. Davies, A. Whiting, Green Chem., 2008, 10, 124-134, DOI: 10.1039/b712008g; b) S. Liu, Y. Yang, X. Liu, A. S. Batsanov and A. Whiting, Eur. J. Org. Chem., 2013, 5692-5700, DOI: 10.1002/ejoc.201300560
5. K. Arnold, B. Davies, D. Hérault, A. Whiting, Angew. Chem., 2008, 47, 2673-2676, DOI: 10.1002/anie.200705643
6. a) H. Charville, D. Jackson, G. Hodges, A. Whiting, M. R. Wilson, Eur. J. Org. Chem., 2011, 5981–5990, DOI: 10.1002/ejoc.201100714; b) S. Arkhipenko, M. T. Sabatini, A. S. Batsanov, V. Karaluka, T. D. Sheppard, H. S. Rzepa and A. Whiting, Chem. Sci., 2018, 9, 1058-1072
7. a) L. Di Bari, S. Guillarme, J. Hanan, A. P. Henderson, J. A. K. Howard, G. Pescitelli, M. R. Probert, P. Salvadori, A. Whiting, Eur. J. Org. Chem., 2007, 5771-5779, DOI: 10.1002/ejoc.200700731; b) P. R. Girling, A. S. Batsanov, A. D. J. Calow, H. C. Shen, A. Whiting, Tetrahedron, 2016, DOI: 10.1016/j.tet.2016.01.006
8. D. Chaiyaveij, A. S. Batsanov, M. A. Fox, T. B. Marder and A. Whiting, J. Org. Chem., 2015, 80, 9518-9543, DOI: 10.1021/acs.joc.5b01470; b) F. Tripoteau, L. Eberlin, M. A. Fox, B. Carboni and A. Whiting, Chem. Commun., 2013, 49, 5414-5416, DOI:10.1039/C3CC42227E
9. a) C. Solé, A. Tatla, J. Mata, A. Whiting, H. Gulyás, E. Fernandez, Chem. Eur. J., 2011, 17, 14248-14257, DOI: 10.1002/chem.201102081; b) A. D. J. Calow, E. Fernández and A. Whiting, Org. Biomol. Chem., 2014, 12, 6121-6127, DOI: 10.1039/C4OB01142B; c) A. Pujol, A. D. J. Calow, A. S. Batsanov and A. Whiting, Org. Biomol. Chem., 2015, 13, 5122-5130, DOI: 10.1039/C4OB02657H
10. A. S. Batsanov, J. P. Knowles, A. Whiting, J. Org. Chem., 2007, 72, 2525-2532, DOI: 10.1021/jo0626010
11. a) J. H. Barnard, C. E. Bridgens, A. Botsanov, E. B. Cartmell, V. B. Christie, J. C. Collings, T. B. Marder, S. Przyborski, C. P. F. Redfern, A. Whiting, Org. Biomol. Chem., 2008, 6, 3497-3507, DOI: 10.1039/b808574a; b) G. Clemens, K. R. Flower, A. P. Henderson, A. Whiting, S. A. Przyborski, M. Jimenez-Hernandez, F. Ball, P. Bassan, G. Cinque and P. Gardner, Mol. BioSyst., 2013, 9, 677-692, DOI: 10.1039/C3MB25505K
12. Patent application: PCT/GB2015/052956
13. D. J. Berrisford, P. A. Lovell, N. R. Suliman, A. Whiting, Chem. Commun, 2005, 5904-5906.
Other recent publications:
Detection and time-tracking activation of a photosensitiser on live single colorectal cancer cells using Raman spectroscopy, J. Gala de Pablo, D. R. Chisholm, C. A. Ambler, S. A. Peyman, A. Whiting and S. D. Evans, Analyst, 2020, DOI: 10.1039/D0AN01023E
Generating skeletal diversity and complexity from boron-substituted 1,3-dienes and enophiles, B. François, L. Eberlin, F. Berrée,A. Whiting and B. Carboni, Eur. J. Org. Chem., 2020, 3282-3293, DOI: 10.1002/ejoc.202000330
Access to fused pyrroles from cyclic 1,3-dienyl boronic esters and arylnitroso compounds, B. François, Benjamin, L. Eberlin, F. Berrée, A. Whiting and B. Carboni, J. Org. Chem., 2020, 85, 5173-5182; DOI: org/10.1021/acs.joc.9b03214
Decay in retinoic acid signalling in varied models of Alzheimer disease and restoration of gene expression with novel receptor acid receptor ligands (RAR-Ms), T. Khatib, D. R. Chisholm, A. Whiting, B. Platt and P. McCaffery, Alzheimers Res. Ther., 2020, 73, 935-954; DOI: 10.3233/JAD-190931
A low temperature, vinylboronate ester-mediated, iterative cross-coupling approach to xanthomonadin polyenyl pigment analogues, K. S. Madden, J. P. Knowles and A. Whiting, Tet., 2019, 75, 130657; DOI: org/10.1016/j.tet.2019.130657
Genomic and non-genomic pathways are both crucial for peak induction of neurite outgrowth by retinoids, T. Khatib, P. Marini, S. Nunna, D. R. Chisholm, A. Whiting, C. Redfern, I. Greig and P. McCaffery, Cell Commun. Signal., 2019, 17:40, DOI: 10.1186/s12964-019-0352-4
CYP26A1 gene promoter is a useful tool for reporting RAR-mediated retinoid activity, R. Zolfaghari, A. Whiting, C. Ross, C.-H. Wei, D. Chisholm and F. Mattie, Anal. Biochem., 2019, 577, 98-109, DOI: 10.1016/j.ab.2019.04.022
A bioluminescence reporter assay for retinoic acid control of translation of the GluR1 subunit of the AMPA glutamate receptor, T. Khatib, B. Müller, A. Whiting, D. Chisholm, C. Redfern and P. McCaffery, Mol. Neurobiol., 2019, DOI: 10.1007/s12035-019-1571-9
Using Nature’s polyenes as templates: Studies of synthetic xanthomonadin analogues and realising their potential as antioxidants, K. S. Madden, H. R. E. Jokhoo, F. D. Conradi, J. P. Knowles, C. W. Mullineaux and A. Whiting, Org. Biomol. Chem., 2019, 17, 3752-3759, DOI: 10.1039/C9OB00275H
Photoactivated cell-killing involving a low molecular weight, donor-acceptor diphenylacetylene, D. R. Chisholm, R. Lamb, T. Pallett, V. Affleck, C. Holden, J. Marrison, P. O’Toole, P. D. Ashton, K. Newling, A. Steffen, A. K. Nelson, C. Mahler, R. Valentine, T. S. Blacker, A. J. Bain, J. Girkin, T. B. Marder, A. Whiting and C. A. Ambler, Chem. Sci.,2019, 10, 4673-4683, DOI: 10.1039/C9SC00199A
A solid-supported phenylboronic acid-based catalyst for direct amidation, Y. Du, T. Barber, S. E. Lim, I. R. Baxendale and A. Whiting, Chem. Comm., 2019,55, 2916-2919, DOI: 10.1039/C8CC09913H
Fluorescent retinoic acid analogues as probes for biochemical and intracellular characterization of retinoid signalling pathways, D. R. Chisholm, C. Tomlinson, G.-L. Zhou, C. Holden, V. Affleck, R. Lamb, K. Newling, P. Ashton, R. Valentine, C. Redfern, J. Erostyak, G. Makkai, C. A. Ambler, A. Whiting and E. Pohl, ACS Chem. Biol., 2019, 14, 369–377, DOI: 10.1021/acschembio.8b00916
Research interests
- Biological chemistry
- Synthetic retinoids
- Asymmetric Catalysis
- Bifunctional Catalysis
- Target and Natural Product Synthesis
Esteem Indicators
- 2000: FRSC: Admitted as Fellow of the Royal Society of Chemistry
Publications
Journal Article
- Hughes, J. G., Chisholm, D. R., Whiting, A., Girkin, J. M., & Ambler, C. A. (2023). Bullseye Analysis: A Fluorescence Microscopy Technique to Detect Local Changes in Intracellular Reactive Oxygen Species (ROS) Production. Microscopy and Microanalysis, 29(2), 529-539. https://doi.org/10.1093/micmic/ozac040
- Waite, R., Adams, C. T., Chisholm, D. R., Sims, C. H. C., Hughes, J. G., Dias, E., …Ambler, C. A. (2023). The antibacterial activity of a photoactivatable diarylacetylene against Gram-positive bacteria. Frontiers in Microbiology, 14, Article 1243818. https://doi.org/10.3389/fmicb.2023.1243818
- Hudhud, L., Chisholm, D. R., Whiting, A., Steib, A., Pohóczky, K., Kecskés, A., …Helyes, Z. (2022). Synthetic Diphenylacetylene-Based Retinoids Induce DNA Damage in Chinese Hamster Ovary Cells without Altering Viability. Molecules, 27(3), Article 977. https://doi.org/10.3390/molecules27030977
- Zhang, Q., Liu, S., Tian, X., Liu, Y., Fan, S., Huang, B., & Whiting, A. (2022). Cu@CuCl-visible light co-catalysed chlorination of C(sp3)–H bonds with MCln solution and photocatalytic serial reactor-based synthesis of benzyl chloride. Green Chemistry, 24(1), 384-393. https://doi.org/10.1039/d1gc03092b
- Dumon, A. S., Rzepa, H. S., Alamillo-Ferrer, C., Bures, J., Procter, R., Sheppard, T. D., & Whiting, A. (2022). A computational tool to accurately and quickly predict 19F NMR chemical shifts of molecules with fluorine–carbon and fluorine–boron bonds. Physical Chemistry Chemical Physics, 24(34), 20409 - 20425. https://doi.org/10.1039/d2cp02317b
- Du, Y., Sari, O., Erdem, S. S., & Whiting, A. (2021). A Bifunctional B,N-Based Asymmetric Catalytic Nitrostyrene-Michael Addition Acting through a 10-Membered Ring Cyclic Transition State. Helvetica Chimica Acta, 104(12), Article e2100199. https://doi.org/10.1002/hlca.202100199
- Tomlinson, C. W., Cornish, K. A., Whiting, A., & Pohl, E. (2021). Structure-functional relationship of cellular retinoic acid binding proteins I and II interacting with natural and synthetic ligands. Acta Crystallographica Section D: Structural Biology, 77(2), 164-175. https://doi.org/10.1107/s2059798320015247
- Chaiyaveij, D., & Whiting, A. (2021). Copper(II)-catalyzed aerobic oxidation of hydrazides to azo intermediates and their Diels–Alder versus ene trapping. ARKIVOC, 2021(10), 64-76. https://doi.org/10.24820/ark.5550190.p011.606
- Perera-Solis, D. D., Zholobenko, V. L., Whiting, A., & Greenwell, H. C. (2021). Heterogeneous ketonic decarboxylation of dodecanoic acid: studying reaction parameters. RSC Advances, 11(56), https://doi.org/10.1039/d1ra06871g
- Gala De Pablo, J., Chisholm, D., Ambler, C., Peyman, S., Whiting, A., & Evans, S. (2020). Detection and Time-Tracking Activation of a Photosensitiser on Live Single Colorectal Cancer Cells Using Raman Spectroscopy. Analyst, 145(17), 5878-5888. https://doi.org/10.1039/d0an01023e
- François, B., Eberlin, L., Berrée, F., Whiting, A., & Carboni, B. (2020). Generating Skeletal Diversity and Complexity from Boron-Substituted 1,3-Dienes and Enophiles. European Journal of Organic Chemistry, 2020(22), 3282-3293. https://doi.org/10.1002/ejoc.202000330
- François, B., Eberlin, L., Berrée, F., Whiting, A., & Carboni, B. (2020). Access to Fused Pyrroles from Cyclic 1,3-Dienyl Boronic Esters and Arylnitroso Compounds. Journal of Organic Chemistry, 85(8), 5173-5182. https://doi.org/10.1021/acs.joc.9b03214
- Khatib, T., Chisholm, D. R., Whiting, A., Platt, B., & McCaffery, P. (2020). Decay in Retinoic Acid Signaling in Varied Models of Alzheimer’s Disease and In-Vitro Test of Novel Retinoic Acid Receptor Ligands (RAR-Ms) to Regulate Protective Genes. Journal of Alzheimer's Disease, 73(3), 935-954. https://doi.org/10.3233/jad-190931
- Chisholm, D. R., Hughes, J. G., Blacker, T. S., Humann, R., Adams, C., Callaghan, D., …Whiting, A. (2020). Cellular localisation of structurally diverse diphenylacetylene fluorophores. Organic and Biomolecular Chemistry, 18(45), 9231-9245. https://doi.org/10.1039/d0ob01153c
- Manohara, G. V., Whiting, A., & Greenwell, C. (2019). Reduced to hierarchy: carbon filament supported mixed metal oxide nanoparticles. ACS Omega, 4(23), 20230-20236. https://doi.org/10.1021/acsomega.9b02534
- Madden, K. S., Knowles, J. P., & Whiting, A. (2019). A low temperature, vinylboronate ester-mediated, iterative cross-coupling approach to xanthomonadin polyenyl pigment analogues. Tetrahedron, 75(45), Article 130657. https://doi.org/10.1016/j.tet.2019.130657
- Perera-Solis, D. D., Pimlott, M., Fidment, E., Whiting, A., & Greenwell, H. C. (2019). Adding Value to Waste Minerals in a Circular Economy Framework: Ochre-Derived Layered Double Hydroxide Catalysts in Fatty Acid Ketonisation. Minerals, 9(11), Article 681. https://doi.org/10.3390/min9110681
- Khatib, T., Whiting, A., Chisholm, D. R., Redfern, C., Müller, B., & McCaffery, P. (2019). A Bioluminescence Reporter Assay for Retinoic Acid Control of Translation of the GluR1 Subunit of the AMPA Glutamate Receptor. Molecular Neurobiology, 56(10), 7074-7084. https://doi.org/10.1007/s12035-019-1571-9
- Mosa, F. A., & Whiting, A. (2019). A Facile Autoxidation of an Allylic Alcohol in Air. International Letters of Chemistry, Physics and Astronomy, 83, 41-47. https://doi.org/10.18052/www.scipress.com/ilcpa.83.41
- Zolfaghari, R., Mattie, F. J., Wei, C., Chisholm, D. R., Whiting, A., & Ross, A. C. (2019). CYP26A1 gene promoter is a useful tool for reporting RAR-mediated retinoid activity. Analytical Biochemistry, 577, 98-109. https://doi.org/10.1016/j.ab.2019.04.022
- Chisholm, D., Lamb, R., Pallett, T., Affleck, V., Holden, C., Marrison, J., …Ambler, C. A. (2019). Photoactivated cell-killing involving a low molecular weight, donor-acceptor diphenylacetylene. Chemical Science, 10(17), 4673-4683. https://doi.org/10.1039/c9sc00199a
- Khatib, T., Marini, P., Nunna, S., Chisholm, D. R., Whiting, A., Redfern, C., …McCaffery, P. (2019). Genomic and non-genomic pathways are both crucial for peak induction of neurite outgrowth by retinoids. Cell Communication and Signaling, 17(1), Article 40. https://doi.org/10.1186/s12964-019-0352-4
- Madden, K. S., Jokhoo, H., Conradi, F., Knowles, J., Mullineaux, C., & Whiting, A. (2019). Using Nature’s polyenes as templates: Studies of synthetic xanthomonadin analogues and realising their potential as antioxidants. Organic and Biomolecular Chemistry, 17(15), 3752-3759. https://doi.org/10.1039/c9ob00275h
- Hou, Y., Ma, J., Yang, H., Anderson, E. A., Whiting, A., & Wu, N. (2019). Palladium-catalysed ligand-free reductive Heck cycloisomerisation of 1,6-en-α-chloro-enamides. Chemical Communications, 55(26), 3733-3736. https://doi.org/10.1039/c9cc00537d
- Chisholm, D., Tomlinson, C., Zhou, G., Holden, C., Affleck, V., Lamb, R., …Pohl, E. (2019). Fluorescent retinoic acid analogues as probes for biochemical and intracellular characterization of retinoid signalling pathways. ACS Chemical Biology, 14(3), 369-377. https://doi.org/10.1021/acschembio.8b00916
- Du, Y., Barber, T., Lim, S. E., Rzepa, H. S., Baxendale, I. R., & Whiting, A. (2019). A solid-supported arylboronic acid catalyst for direct amidation. Chemical Communications, 55(20), 2916-2919. https://doi.org/10.1039/c8cc09913h
- Tomlinson, C. W., Chisholm, D. R., Valentine, R., Whiting, A., & Pohl, E. (2018). A novel fluorescence competition assay for retinoic acid binding proteins. ACS Medicinal Chemistry Letters, 9(12), 1297–1300-1300. https://doi.org/10.1021/acsmedchemlett.8b00420
- Madden, K., Laroche, B., David, S., Batsanov, A., Thompson, D., Knowles, J., & Whiting, A. (2018). Approaches to Styrenyl Building Blocks for the Synthesis of Polyene Xanthomonadin and its Analogues. European Journal of Organic Chemistry, 2018(38), 5312-5322. https://doi.org/10.1002/ejoc.201800540
- Liu, S., Zhang, Q., Tian, X., Fan, S., Huang, J., & Whiting, A. (2018). Highly selective halogenation of unactivated C(sp3)–H with NaX under co-catalysis of visible light and Ag@AgX. Green Chemistry, 20(20), 4729-4737. https://doi.org/10.1039/c8gc02628a
- Rzepa, H., Arkhipenko, S., Wang, E., Batsanov, A., Sabatini, M., Karaluka, V., …Sheppard, T. (2018). An accessible method for DFT calculation of 11B NMR shifts of organoboron compounds. Journal of Organic Chemistry, 83(15), 8020-8025. https://doi.org/10.1021/acs.joc.8b00859
- Haffez, H., Chisholm, D., Tatum, N., Valentine, R., Redfern, C., Pohl, E., …Przyborski, S. (2018). Probing biological activity through structural modelling of ligand-receptor interactions of 2,4-disubstituted thiazole retinoids. Bioorganic and Medicinal Chemistry, 26(8), 1560-1572. https://doi.org/10.1016/j.bmc.2018.02.002
- Eberlin, L., Macé, A., Batsanov, A., Carboni, B., & Whiting, A. (2018). A Dienyl Boronate‐Aryl Nitroso Ene Reaction Entry to C‐Pyrrolyl Nitrones and Subsequent Conversion to Isoxazolidines. ChemistrySelect, 3(16), 4557-4561. https://doi.org/10.1002/slct.201800945
- Haffez, H., Khatib, T., McCaffery, P., Przyborski, S., Redfern, C., & Whiting, A. (2018). Neurogenesis in Response to Synthetic Retinoids at Different Temporal Scales. Molecular Neurobiology, 55(3), 1942-1950. https://doi.org/10.1007/s12035-017-0440-7
- Arkhipenko, S., Batsanov, A., Sabatini, M., Karaluka, V., Sheppard, T., Rzepa, H., & Whiting, A. (2018). Mechanistic insights into boron-catalysed direct amidation reactions. Chemical Science, 9(4), 1058-1072. https://doi.org/10.1039/c7sc03595k
- Pujol, A., & Whiting, A. (2017). Double Diastereoselective Approach to Chiral syn- and anti-1,3-Diol Analogues through Consecutive Catalytic Asymmetric Borylations. Journal of Organic Chemistry, 82(14), 7265-7279. https://doi.org/10.1021/acs.joc.7b00854
- Haffez, H., Chisholm, D., Valentine, R., Pohl, E., Redfern, C., & Whiting, A. (2017). The molecular basis of the interactions between synthetic retinoic acid analogues and the retinoic acid receptors. MedChemComm, 8(3), 578-592. https://doi.org/10.1039/c6md00680a
- Chisholm, D. R., Valentine, R., Pohl, E., & Whiting, A. (2016). Conjugate Addition of 3-Buytn-2-one to Anilines in Ethanol: Alkene Geometric Insights through In Situ FTIR Monitoring. Journal of Organic Chemistry, 81(17), 7557-7565. https://doi.org/10.1021/acs.joc.6b01110
- Chisholm, D., Zhou, G., Pohl, E., Valentine, R., & Whiting, A. (2016). Practical synthetic strategies towards lipophilic 6-iodotetrahydroquinolines and -dihydroquinolines. Beilstein Journal of Organic Chemistry, 12, 1851-1862. https://doi.org/10.3762/bjoc.12.174
- Girling, P., Batsanov, A., Calow, A., Shen, H., & Whiting, A. (2016). Alternative tandem cyclisation pathways in the reaction between imines and enones,. Tetrahedron, 72(8), 1105-1113. https://doi.org/10.1016/j.tet.2016.01.006
- Chaiyaveij, D., Batsanov, A., Fox, M., Marder, T., & Whiting, A. (2015). An experimental and computational approach to understanding the reactions of acyl nitroso compounds in [4+2]-cycloadditions. Journal of Organic Chemistry, 80(19), 9518-9534. https://doi.org/10.1021/acs.joc.5b01470
- Whiting, A., Madden, K. S., David, S., & Knowles, J. P. (2015). Heck-Mizoroki Coupling of Vinyliodide and Applications in The Synthesis of Dienes and Trienes. Chemical Communications, 51(57), 11409-11412. https://doi.org/10.1039/c5cc03273c
- Eberlin, L., Carboni, B., & Whiting, A. (2015). Regioisomeric and substituent effects upon the outcome of the reaction of 1-borodienes with nitrosoarene compounds. Journal of Organic Chemistry, 80(13), 6574-6583. https://doi.org/10.1021/acs.joc.5b00593
- Liu, S., Zhang, Q., Li, H., Yang, Y., Tiana, X., & Whiting, A. (2015). A visible light induced α-H chlorination of alkylarenes with inorganic chloride under nanoAg@AgCl. Chemistry - A European Journal, 21(27), 9671-9675. https://doi.org/10.1002/chem.201501439
- Pujol, A., Calow, A., Batsanov, A., & Whiting, A. (2015). One-pot catalytic asymmetric borylation of unsaturated aldehyde-derived imines; functionalisation to homoallylic boronate carboxylate ester derivatives. Organic and Biomolecular Chemistry, 13(18), 5122-5130. https://doi.org/10.1039/c4ob02657h
- Calow, A., Carbó, J., Cid, J., Fernández, E., & Whiting, A. (2014). Understanding α,β-unsaturated imine formation from amine additions to α,β-unsaturated aldehydes and ketones: an analytical and theoretical investigation. Journal of Organic Chemistry, 79(11), 5163-5172. https://doi.org/10.1021/jo5007366
- Batsanov, A., Georgiou, I., Girling, P., Pommier, L., Shen, H., & Whiting, A. (2014). Asymmetric synthesis and application of homologous pyrroline-2-alkylboronic acids: Identification of the B-N distance for eliciting bifunctional catalysis of an asymmetric aldol reaction. Asian Journal of Organic Chemistry, 3(4), 470-479. https://doi.org/10.1002/ajoc.201300127
- Lopez-Real, R., Budge, J., Marder, T., Whiting, A., Hunt, P., & Przyborski, S. (2014). Application of Synthetic Photostable Retinoids Induces Novel Limb and Facial Phenotypes During Chick Embryogenesis In Vivo. Journal of Anatomy, 224(4), 392-411. https://doi.org/10.1111/joa.12147
- La Cascia, E., Sanz, X., Bo, C., Whiting, A., & Fernandez, E. (2014). Asymmetric metal free β-boration of α,β-unsaturated imines assisted by (S)-MeBoPhoz. Organic and Biomolecular Chemistry, 13(5), 1328-1332. https://doi.org/10.1039/c4ob02478h
- Madden, K., Mosa, F., & Whiting, A. (2014). Non-isoprenoid polyene natural products - structures and synthetic strategies. Organic and Biomolecular Chemistry, 12, 7877-7899. https://doi.org/10.1039/c4ob01337a
- Clemens, G., Flower, K., Gardner, P., Henderson, A., Knowles, J., Marder, T., …Przyborski, S. (2013). Design and biological evaluation of synthetic retinoids: Probing length vs. stability vs. activity. Molecular bioSystems, 9(12), 3124-3134. https://doi.org/10.1039/c3mb70273a
- Ilyashenko, G., Al-Safadi, R., Donnan, R., Dubrovka, R., Pancholi, J., Watkinson, M., & Whiting, A. (2013). Facile synthesis of a 1,1’-desymmetrised ferrocene backbone and its one-pot double-“click” functionalisation. RSC Advances, 3(38), 17081-17087. https://doi.org/10.1039/c3ra43054e
- Calow, A., Batsanov, A., Pujol, A., Solé, C., Fernández, E., & Whiting, A. (2013). A selective transformation of enals into chiral γ-amino alcohols. Organic Letters, 15(18), 4810-4813. https://doi.org/10.1021/ol4022029
- Liu, S., Yang, Y., Liu, X., Ferdousi, F., Batsanov, A., & Whiting, A. (2013). Direct Amidation of Amino Acid Derivatives Catalyzed by Arylboronic Acids: Applications in Dipeptide Synthesis. European Journal of Organic Chemistry, 2013(25), 5692-5700. https://doi.org/10.1002/ejoc.201300560
- Calow, A., Solé, C., Whiting., A., & Fernández, E. (2013). Base-free β-boration of α,β-unsaturated imines catalysed by Cu2O with concurrent enhancement of asymmetric induction. ChemCatChem, 5(8), 2233-2239. https://doi.org/10.1002/cctc.201300113
- Tripoteau, F., Eberlin, L., Fox, M., Carboni, B., & Whiting, A. (2013). A novel, efficient synthesis of N-aryl pyrroles via reaction of 1-boronodienes with arylnitroso compounds. Chemical Communications, 49(47), 5414-5416. https://doi.org/10.1039/c3cc42227e
- Zhou, G., Tams, D., Marder, T., Valentine, R., Whiting, A., & Przyborski, S. (2013). Synthesis and applications of 2,4-disubstituted thiazoles derivatives as small molecule modulators of cellular development. Organic and Biomolecular Chemistry, 11(14), 2323-2334. https://doi.org/10.1039/c3ob00005b
- Clemens, G., Flower, K., Henderson, A., Whiting, A., Przyborski, S., Jimenez-Hernandez, M., …Gardner, P. (2013). The action of all-trans-retinoic acid (ATRA) and synthetic retinoid analogues (EC19 and EC23) on human pluripotent stem cells differentiation investigated using single cell infrared microspectroscopy. Molecular bioSystems, 9(4), 677-692. https://doi.org/10.1039/c3mb25505k
- Georgiou, I., & Whiting, A. (2012). Mechanism and optimisation of the homoboroproline bifunctional catalytic asymmetric aldol reaction: Lewis acid tuning through in situ esterification. Organic and Biomolecular Chemistry, 10(12), 2422-2430. https://doi.org/10.1039/c2ob06872a
- Calow;, A., Solé;, C., Fernandez;, E., & Whiting;, A. (2012). Novel transformation of α,β-unsaturated aldehydes and ketones to γ-amino alcohols or 1,3-oxazines via a 4 or 5 step, one-pot sequence. Chemical Communications, 48(93), https://doi.org/10.1039/c2cc36129a
- Na;, W., Messinis;, A., Batsanov;, A., Zhen;, Y., Whiting;, A., & Marder;, T. (2012). Palladium(II)-catalysed tandem cyclisation of electron-deficient aromatic enynes. Chemical Communications, 48(80), 9986-9988. https://doi.org/10.1039/c2cc35114e
- Liu;, S., Yang;, Y., Zhen;, X., Li;, J., He;, H., Feng;, J., & Whiting;, A. (2012). Stereoselective synthesis and rearrangement-fragmentation of arylidene N-alkoxydiketopiperazines. Organic and Biomolecular Chemistry, 10(3), 663-670. https://doi.org/10.1039/c1ob06471a
- Girling, P., Batsanov, A., Shen, H., & Whiting, A. (2012). A multicomponent formal [1+2+2+1]-cycloaddition for the synthesis of dihydropyridines. Chemical Communications, 48(40), 4893-4895. https://doi.org/10.1039/c2cc31495a
- Georgiou;, I., & Whiting;, A. (2012). An efficient enantioselective (R)-homoboroproline from (S)-proline using borylation approach. European Journal of Organic Chemistry, 2012(22), 4110-4113. https://doi.org/10.1002/ejoc.201200652
- Charville, H., Jackson, D., Hodges, G., Whiting, A., & Wilson, M. (2011). The Uncatalyzed Direct Amide Formation Reaction – Mechanism Studies and the Key Role of Carboxylic Acid H-Bonding. European Journal of Organic Chemistry, 2011(30), 5981-5990. https://doi.org/10.1002/ejoc.201100714
- Solé, C., Whiting, A., Gulyás, H., & Fernández, E. (2011). Highly Enantio- and Diastereoselective Synthesis of γ-Amino Alcohols from α,β-Unsaturated Imines through a One-Pot β-Boration/Reduction/Oxidation Sequence. Advanced Synthesis & Catalysis, 353(2-3), 376-384. https://doi.org/10.1002/adsc.201000842
- and Hydrazides to Acyl-Nitroso and Azo Intermediates, and Their Diels_Alder Trapping. Organic Letters, 13(13), 3442-3445. https://doi.org/10.1021/ol201188d
- Knowles;, J., O’Connor;, V., & Whiting;, A. (2011). Studies towards the synthesis of the northern polyene of viridenomycin and synthesis of Z-double bond analogues. Organic and Biomolecular Chemistry, 9(6), 1876-1886. https://doi.org/10.1039/c0ob00977f
- Solé;, C., Tatla;, A., Mata;, J., Whiting;, A., Gulyás;, H., & Fernandez;, E. (2011). Catalytic 1,3-difunctionalization of organic backbones via a highly stereoselective, one-pot, boron conjugate-addition/reduction/oxidation process,. Chemistry - A European Journal, 17(50), https://doi.org/10.1002/chem.201102081
- Arnold, K., Batsanov, A., Davies, B., Grosjean, C., Schütz, T., Whiting, A., & Zawatzkya, K. (2008). The first example of enamine–Lewis acid cooperative bifunctional catalysis: application to the asymmetric Aldol reaction. Chemical Communications, 3879-3881. https://doi.org/10.1039/b806779a
- Aelvoet, K., Batsanov, A., Blatch, A., Grosjean, C., Patrick, L., Smethurst, C., & Whiting, A. (2008). A catalytic aldol reaction and condensation through in situ boron "ate"-complex enolate generation in water. Angewandte Chemie International Edition, 47(4), 768-770. https://doi.org/10.1002/anie.200704293
- Arnold, K., Davies, B., Hérault, D., & Whiting, A. (2008). Asymmetric Direct Amide Synthesis by Kinetic Amine Resolution: A Chiral Bifunctional Aminoboronic Acid Catalyzed Reaction between a Racemic Amine and an Achiral Carboxylic Acid. Angewandte Chemie International Edition, 47(14), 2673-2676. https://doi.org/10.1002/anie.200705643
- Herault, D., Aelvoet, K., Blatch, A., Al-Majid, A., Smethurst, C., & Whiting, A. (2007). Benzimidazole nitrogen-directed, regiocontrolled, lithiation of ferrocenyl- and phenyl-N-n-butylbenzimidazoles. Journal of Organic Chemistry, 72(1), 71-75. https://doi.org/10.1021/jo061639%2B
- Knowles, J., & Whiting, A. (2007). The effects of ring size and substituents on the rates of acid-catalysed hydrolysis of five- and six-membered ring cyclic ketone acetals. European Journal of Organic Chemistry, 2007(20), 3365-3368. https://doi.org/10.1002/ejoc.200700244
- Batsanov, A., Knowles, J., & Whiting, A. (2007). Mechanistic studies on the Heck-Mizoroki cross-coupling reaction of a hindered vinylboronate ester as a key approach to developing a highly stereoselective synthesis of a Cl-C7 Z,Z,E-triene synthon for viridenomycin. Journal of Organic Chemistry, 72(7), 2525-2532. https://doi.org/10.1021/jo0626010
- Blatch, A., Howard, J., Probert, M., Smethurst, C., & Whiting, A. (2006). tert-Butyl N-(phosphinoyloxy)carbamate. Acta crystallographica. Section E, 62(11), o5346-o5348. https://doi.org/10.1107/s1600536806044783
- Blatch, A., Howard, J., Probert, M., & Whiting, A. (2006). N-(diphenylphosphinoyl)hydroxylamine. Acta crystallographica. Section E, 62(11), o5343-o5345. https://doi.org/10.1107/s1600536806044862
- Arnold, K., Davies, B., Giles, R., Grosjean, C., Smith, G., & Whiting, A. (2006). To catalyze or not to catalyze? Insight into direct amide bond formation from amines and carboxylic acids under thermal and catalyzed conditions. Advanced Synthesis & Catalysis, 348(7-8), 813-820. https://doi.org/10.1002/adsc.200606018
- Blatch, A., Chetina, O., Howard, J., Patrick, L., Smethurst, C., & Whiting, A. (2006). Synthesis and structure of bifunctional N-alkylbenzimidazole phenylboronate derivatives. Organic and Biomolecular Chemistry, 4(17), 3297-3302. https://doi.org/10.1039/b607127a
- Mears, R., Sailes, H., Watts, J., & Whiting, A. (2006). A stereoselective remote homochiral boronate ester-mediated aldol reaction. ARKIVOC, 2006(1), 95-103. https://doi.org/10.3998/ark.5550190.0007.111
- Whiting, A., & Walton, S. (2006). Unexpected exothermic reaction between thioacetic acid and DMSO. Organic Process Research and Development, 10(4), 846-846. https://doi.org/10.1021/op060109c
- Batsanov, A., Lightfoot, A., Twiddle, S., & Whiting, A. (2006). Bis(2,6-dimethylpyridyl)iodonium dibromoiodate. Acta crystallographica. Section E, E62, o901-o902. https://doi.org/10.1107/s1600536806003680
- Greenwell, H., Bowden, A., Chen, B., Boulet, P., Evans, J., Coveney, P., & Whiting, A. (2006). Intercalation and in situ polymerization of poly(alkylene oxide) derivatives within M+-montmorillonite (M = Li, Na, K). Journal of materials chemistry, 16(11), 1082-1094. https://doi.org/10.1039/b505217c
- Blatch, A., Howard, J., Probert, M., Smethurst, C., & Whiting, A. (2006). Potassium 4-nitrophenylsulfonate monohydrate. Acta crystallographica. Section E, 62(4), m741-m743. https://doi.org/10.1107/s1600536806007835
- Coghlan, S., Giles, R., Howard, J., Patrick, L., Probert, M., Smith, G., & Whiting, A. (2005). Synthesis and structure of potential Lewis acid-Lewis base bifunctional catalysts: 2-N,N-diisopropylaminophenylboronate derivatives. Journal of Organometallic Chemistry, 690(21-22), 4784-4793
- Hamlin, J., & Whiting, A. (2005). An insight into the mechanism of the cellulose dyeing process, part 2: Simulation of aggregation, solvent and additive effects upon azo-linked aromatics and dyes. Molecular Simulation, 31(8), 605-612
- Chen, B., Bowden, A., Greenwell, H., Boulet, P., Coveney, P., Whiting, A., & Evans, J. (2005). Morphology and elastic modulus of novel poly oligo(ethylene glycol) diacrylate -montmorillonite nanocomposites. Journal of Polymer Science Part B: Polymer Physics, 43(14), 1785-1793
- Greenwell, H., Harvey, M., Boulet, P., Bowden, A., Coveney, P., & Whiting, A. (2005). Interlayer structure and bonding in nonswelling primary amine intercalated clays. Macromolecules, 38(14), 6189-6200. https://doi.org/10.1021/ma0503817
- Lightfoot, A., Twiddle, S., & Whiting, A. (2005). A stereoselective synthesis of 1,6-diphenyl-1,3,5-hexatrienes utilising 4,4,6-trimethyl-2-vinyl-1,3,2-dioxaborinane as a two-carbon alkenyl building block. Organic and Biomolecular Chemistry, 3(17), 3167-3172. https://doi.org/10.1039/b507900d
- Di Bari, L., Guillarme, S., Hermitage, S., Jay, D., Pescitelli, G., & Whiting, A. (2005). Absolute stereochemistry assignment of N-phosphorylimine-derived aza-Diels-Alder adducts with TDDFF CD calculations. Chirality, 17(6), 323-331
- Batsanov, A., Howard, J., Lightfoot, A., Twiddle, S., & Whiting, A. (2005). Stereoselective chloro-deboronation reactions induced by substituted pyridine-iodine chloride complexes. European Journal of Organic Chemistry, 1876-1883
- Lightfoot, A., Twiddle, S., & Whiting, A. (2005). 4,4,6-trimethyl-2-vinyl-1,3,2-dioxaborinane: An efficient and selective 2-carbon building block for vinylboronate Suzuki-Miyaura coupling reactions. Synlett: Accounts and Rapid Communications in Synthetic Organic Chemistry, 529-531
- Berrisford, D., Lovell, P., Sulliman, N., & Whiting, A. (2005). Latent reactive groups unveiled through equilibrium dynamics and exemplified in crosslinking during film formation from aqueous polymer colloids. Chemical Communications, 5904-5906
- Lightfoot, A., Twiddle, S., & Whiting, A. (2004). On the mechanism and origin of the stereoselectivity in iodo-deboronation and chloro-deboronation of hindered alkenyl boronate esters using either ICI-NaOMe or ICI-pyridine. Tetrahedron Letters, 45(46), 8557-8561
- Bowden, A., Whiting, A., Coveney, P., Evans, J., Greenwell, H., Boulet, P., & Chen, B. (2004). ATRP polymerized PEG-methyl ether methacrylatemontmorillonite nanocomposites
- Hermitage, S., Howard, J., Jay, D., Pritchard, R., Probert, M., & Whiting, A. (2004). Mechanistic studies on the formal aza-Diels-Alder reactions of N-aryl imines: evidence for the non-concertedness under Lewis-acid catalysed conditions. Organic and Biomolecular Chemistry, 2(17), 2451-2460. https://doi.org/10.1039/b407293f
- Maw, G., Thirsk, C., Toujas, J., Vaultier, M., & Whiting, A. (2004). Preparation of an advanced phenylglycine-derived intermediate en route to the southern hemisphere tetraene of viridenomycin. Synlett: Accounts and Rapid Communications in Synthetic Organic Chemistry, 1183-1186
- Di Bari, L., Guillarme, S., Hermitage, S., Howard, J., Jay, D., Pescitelli, G., …Yufit, D. (2004). Lewis acid-catalysed aza-Diels-Alder versus Mannich reactions of N-diethyl phosphoryl imino dienophiles with oxygenated dienes and application of a chiral lewis acid. Synlett: Accounts and Rapid Communications in Synthetic Organic Chemistry, 708-710
- Guillarme, S., & Whiting, A. (2004). Unexpected temperature, time and solvent effects in the catalytic asymmetric aza-Diels-Alder reaction of an ethyl glyoxylate-derived N-aryl imine with Danishefsky's diene catalysed by a BINOL-zinc complex. Synlett: Accounts and Rapid Communications in Synthetic Organic Chemistry, 711-713
- Bundu, A., Guillarme, S., Hannan, J., Wan, H., & Whiting, A. (2003). A parallel combinatorial approach to locating homochiral Lewis acid catalysts for the asymmetric aza-Diels-Alder reaction of an imino dienophile (vol 39, pg 8905, 1998). Tetrahedron Letters, 44(42), 7849-7850
- Lightfoot, A., Maw, G., Thirsk, C., Twiddle, S., & Whiting, A. (2003). 4,4,6-trimethyl-2-vinyl-1,3,2-dioxaborinane: a superior 2-carbon building block for vinylboronate Heck couplings. Tetrahedron Letters, 44(41), 7645-7648
- Giles, R., Howard, J., Patrick, L., Probert, M., Smith, G., & Whiting, A. (2003). Synthesis and structure of potential Lewis acid-Lewis base bifunctional catalysts: 1-N,N-dimethylamino-8-borononaphthalene derivatives. Journal of Organometallic Chemistry, 680(1-2), 257-262
- Pulacchini, S., Sibbons, K., Shastri, K., Motevalli, M., Watkinson, M., Wan, H., …Lightfoot, A. (2003). Synthesis of C-2-symmetric aza- and azaoxa-macrocyclic ligands derived from (1R,2R)-1,2-diaminocyclohexane and their applications in catalysis. Dalton Transactions, 2043-2052
- Boulet, P., Bowden, A., Coveney, P., & Whiting, A. (2003). Combined experimental and theoretical investigations of clay polymer nanocomposites: intercalation of single bifunctional organic compounds in Na+-montmorillonite and Na+-hectorite clays for the design of new materials. Journal of materials chemistry, 13(10), 2540-2550
- Flower, K., Hamlin, J., & Whiting, A. (2002). An insight into the mechanism of the cellulose dyeing process, part 1: Modelling azo-systems in azo-linked aromatics and dyes. Molecular Simulation, 28(12), 1031-1047
- Hermitage, S., Jay, D., & Whiting, A. (2002). Evidence for the non-concerted 4+2 -cycloaddition of N-aryl imines when acting as both dienophiles and dienes under Lewis acid-catalysed conditions. Tetrahedron Letters, 43(52), 9633-9636
- Patrick, L., & Whiting, A. (2002). Synthesis of some polymerisable fluorescent dyes. Dyes and Pigments, 55(2-3), 123-132
- Whiting, A., Wan, H., Lightfoot, A., & Flower, K. (2002). New ruthenium based methods for the in situ generation of acyl nitroso dienophiles
- Whiting, A., & Wan, H. (2002). New approaches to catalytic asymmetric nitroso diels-alder reactions
- Whiting, A., Patrick, L., Giles, R., & Coghlan, S. (2002). Modular bifunctional Lewis acid-Lewis base catalysts
- Bowden, A., Whiting, A., & Coveney, P. (2002). Preparation and analysis of poly(oxyalkylene)-clay nanocomposites
- Bosbach, D., Coveney, P., Griffin, J., Putnis, A., Risthaus, P., Stackhouse, S., & Whiting, A. (2002). The rational design, synthesis and demonstration of the recognition and binding of a diaza-dioxa-12-crown-4 diphosphonate macrocycle to all crystal growth faces of barium sulfate. Journal of the Chemical Society. Perkin transactions II, 2(7), 1238-1245. https://doi.org/10.1039/b203285f
- Brooke, G., Cameron, N., MacBride, J., & Whiting, M. (2002). The synthesis of oligomers related to poly(ethyleneglycol terephthalate). Polymer, 43(4), 1139-1154
- Jay, D., Whiting, A., Yufit, D., Howard, J., & Hermitage, S. (2002). p-Tolylsulfonyl cyanide. Acta crystallographica. Section B, Structural science, 58, o191-o193
- Thirsk, C., & Whiting, A. (2002). Polyene natural products. Journal of the Chemical Society. Perkin transactions 1, 999-1023
- Flower, K., Lightfoot, A., Wan, H., & Whiting, A. (2002). The development and application of ruthenium catalysed oxidations of a hydroxamic acid and in situ Diels-Alder trapping of the acyl nitroso derivative. Journal of the Chemical Society. Perkin transactions 1, 2058-2064
- Lightfoot, A., Pritchard, R., Wan, H., Warren, J., & Whiting, A. (2002). A novel scandium ortho-methoxynitrosobenzene-dimer complex: mechanistic implications for the nitroso-Diels-Alder reaction. Chemical Communications, 2072-2073
- Thirsk, C., Hawkes, G., Kroemer, R., Liedl, K., Loerting, T., Nasser, R., …Whiting, A. (2002). The structure, modelling and dynamics of 2,7-diisopropoxy-1,8-diarylnaphthalenes. Journal of the Chemical Society-Perkin Transactions 2, 1510-1519

