Staff profile
Professor Steven Cobb
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42086 |
| Director in the Biophysical Sciences Institute | |
| Director in the Biophysical Sciences Institute | |
| Fellow of the Wolfson Research Institute for Health and Wellbeing |
Biography
Steven Cobb carried out his PhD with Professor David O’Hagan at St. Andrews University (2001-2005). His work focused on the biosynthesis of fluorinated natural products, in the bacteria Streptomyces cattleya. The highlight of this research was the identification of the first ever naturally-occurring C-F bond forming enzyme (Nature, 2002, 416, 279). Steven was awarded an Alberta Heritage Foundation for Medical Research Fellowship and moved to the University of Alberta, to work with Professor John Vederas, FRS. During this time he was introduced to the field of peptide chemistry and he worked on the development of new peptide based antibiotics. In October 2007 Steven moved to the Chemistry Department at Durham University as a temporary lecturer, and from this position he was awarded a prestigious Ramsay Memorial Trust Research Fellowship (2008–2010). In 2010 he was appointed to the position of Lecturer and since then he has attracted research funding from various competitive sources as a PI including the Royal Society, the EPSRC, The Wellcome Trust, The Leverhulme Trust, UK industry and he was recently the successful PI on a Bill and Melinda Gates Foundation Grant (one of only 100 grants award worldwide from over 2100 applications).
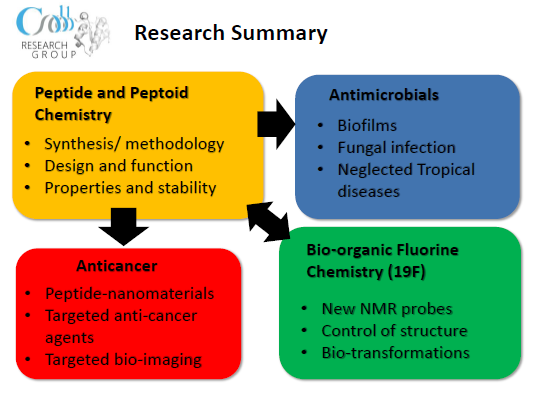
Research Interests
Our group uses a range of methods and techniques in synthetic organic, peptoid and peptide chemistry to tackle interesting and challenging biological problems.
For further details, see my personal web pages
Recent Publications
D. Gimenez, C.A. Mooney, A. Dose, G. Sandford, C. R. Coxon and S.L. Cobb* “The application of perfluoroheteroaromatic reagents in the preparation of modified peptide systems” Org. Biomol. Chem., 2017, 15, 4086-4095
D. Gimenez, A. Dose, N.L. Robson, G. Sandford, S. L. Cobb* and C. R. Coxon* “2,2,2-Trifluoroethanol as a solvent to control nucleophilic peptide arylation” Org. Biomol. Chem. 2017, 15,4081-4085
H.L. Bolt, G.A. Eggimann, C.A.B. Jahoda, R. N. Zuckermann, G. J. Sharples*, S.L. Cobb* “Exploring the links between peptoid antibacterial activity and toxicity” MedChemComm. 2017, 8(5), 886-896
Luoa Y., Bolt HL., Eggimann GA., McAuley DF., McMullan R., Curran T., Cobb SL*, Lundy FT* "Peptoid Efficacy against Polymicrobial Biofilms Determined by Using Propidium Monoazide-Modified Quantitative PCR" ChemBioChem, 2017, 18, 111– 118
L. Jiang, R. Lan, T. Huang, C.-F. Chan, H. Li, S. Lear, J. Zong, W.-Y. Wong, M. M.-Lan Lee, B. D. Chan, W.-L. Chan, W.-S. Lo, N.-K. Mak, M. L. Lung, G. S. Taylor, Z.-X. Bian, W. C. S. Tai, G.-L. Law, W.-T. Wong, S.L. Cobb,* and K.-L. Wong,* “EBNA1-targeted probe for the imaging and growth inhibition of tumours associated with the Epstein–Barr virus”, Nat. Biomed. Eng., 2017, 0042 [Highlighted in Nature Biomedical Engineering, 2017, 0059 and in Cell Chemical Biology, 2017, 647-648]
Luoa Y., Bolt HL., Eggimann GA., McAuley DF., McMullan R., Curran T., Cobb SL*, Lundy FT* "Peptoid Efficacy against Polymicrobial Biofilms Determined by Using Propidium Monoazide-Modified Quantitative PCR" ChemBioChem, 2017, 18, 111– 118
Lear S., Munshi T., Hudson AS., Hatton C., Clardy J., Mosely JA., Bull TJ., Sit CS.,* and S. L. Cobb* "Total Chemical Synthesis of Lassomycin and Lassomycin-amide" Org. Biomol. Chem. 2016, 14, 4534-4541
Bolt HL, Eggimann GA, Denny PW,* and Cobb SL.,* Enlarging the chemical space of anti-leishmanials: a structure-activity relationship study of peptoids against Leishmania mexicana, a causative agent of cutaneous leishmaniasis” MedChemComm. 2016, 7, 799-805
Research interests
- Peptide Drug Design and Delivery
- Peptidomimetics
- Bioorganic Fluorine Chemistry
Publications
Journal Article
- Stewart, L. J., Hong, Y., Holmes, I. R., Firth, S. J., Ahmed, Y., Quinn, J., …Djoko, K. Y. (2023). Salivary Antimicrobial Peptide Histatin-5 Does Not Display Zn(II)-Dependent or -Independent Activity against Streptococci. ACS Infectious Diseases, 9(3), 631-642. https://doi.org/10.1021/acsinfecdis.2c00578
- Beardmore, L. N., Cobb, S. L., & Brittain, W. D. (2022). One-pot ester and thioester formation mediated by pentafluoropyridine (PFP). Organic and Biomolecular Chemistry, 20(41), 8059-8064. https://doi.org/10.1039/d2ob01268e
- Wang, S., Brittain, W. D., Zhang, Q., Lu, Z., Tong, M. H., Wu, K., …Deng, H. (2022). Aminoacyl chain translocation catalysed by a type II thioesterase domain in an unusual non-ribosomal peptide synthetase. Nature Communications, 13(1), https://doi.org/10.1038/s41467-021-27512-0
- Gimenez, D., Phelan, A., Murphy, C. D., & Cobb, S. L. (2021). Fengycin A Analogues with Enhanced Chemical Stability and Antifungal Properties. Organic Letters, 23(12), 4672-4676. https://doi.org/10.1021/acs.orglett.1c01387
- Karunakaran, K., Sundriyal, S., Perera, H., Cobb, S. L., & Denny, P. W. (2021). Quantitative Proteomics Reveals that Hsp90 Inhibition Dynamically Regulates Global Protein Synthesis in Leishmania mexicana. mSystems, https://doi.org/10.1128/msystems.00089-21
- Brittain, W. D., & Cobb, S. L. (2021). Carboxylic Acid Deoxyfluorination and One-Pot Amide Bond Formation Using Pentafluoropyridine (PFP). Organic Letters, 23(15), https://doi.org/10.1021/acs.orglett.1c01953
- Toole, J., Bolt, H. L., Marley, J. J., Patrick, S., Cobb, S. L., & Lundy, F. T. (2021). Peptoids with Antibiofilm Activity against the Gram Negative Obligate Anaerobe, Fusobacterium nucleatum. Molecules, 26(16), Article 4741. https://doi.org/10.3390/molecules26164741
- Zong, J., Cobb, S. L., & Cameron, N. R. (2021). Preparation and thermally-induced self-assembly behaviour of elastin-like peptide side-chain polymer-gold nanoparticle (ESP-GNP) hybrids. ARKIVOC, 2021(6), 131 - 147. https://doi.org/10.24820/ark.5550190.p011.589
- Lloyd, C. M., Colgin, N., & Cobb, S. L. (2020). Current Synthetic Routes to Peptidyl Mono-Fluoromethyl Ketones (FMKs) and Their Applications. Molecules, 25(23), Article 5601. https://doi.org/10.3390/molecules25235601
- Brittain, W. D., Lloyd, C. M., & Cobb, S. L. (2020). Synthesis of Complex Unnatural Fluorine-Containing Amino Acids. Journal of Fluorine Chemistry, 239, Article 109630. https://doi.org/10.1016/j.jfluchem.2020.109630
- Bicker, K. L., & Cobb, S. L. (2020). Recent advances in the development of anti-infective peptoids. Chemical Communications, 56(76), 11158-11168. https://doi.org/10.1039/d0cc04704j
- Maguire, O. R., Taylor, B., Higgins, E. M., Rees, M., Cobb, S. L., Simpkins, N. S., …O'Donoghue, A. C. (2020). Unusually high α-proton acidity of prolyl residues in cyclic peptides. Chemical Science, 11(29), 7722-7729. https://doi.org/10.1039/d0sc02508a
- Brittain, W. D. G., & Cobb, S. L. (2020). Protecting Group Controlled Remote Regioselective Electrophilic Aromatic Halogenation Reactions. Journal of Organic Chemistry, 85(11), 6862-6871. https://doi.org/10.1021/acs.joc.9b03322
- Maguire, O. R., Zhu, J., Brittain, W. D., Hudson, A. S., Cobb, S. L., & O’Donoghue, A. C. (2020). N-Terminal speciation for native chemical ligation. Chemical Communications, 56(45), 6114-6117. https://doi.org/10.1039/d0cc01604g
- Zhang, J., Dai, L., Webster, A. M., Chan, W. T. K., Cobb, S. L., & Law, G. (2020). Unusual Magnetic Field Responsive Circularly Polarized Luminescence Probes with Highly Emissive Chiral Eu(III) Complexes. Angewandte Chemie International Edition, 60(2), 1004-1010. https://doi.org/10.1002/anie.202012133
- Jiang, L., Lung, H. L., Huang, T., Lan, R., Zha, S., Chan, L. S., …Wong, K. (2019). Reactivation of Epstein–Barr virus by a dual-responsive fluorescent EBNA1-targeting agent with Zn2+-chelating function. Proceedings of the National Academy of Sciences, 116(52), 26614-26624. https://doi.org/10.1073/pnas.1915372116
- Brittain, W. D., & Cobb, S. L. (2019). Tetrafluoropyridyl (TFP): a general phenol protecting group readily cleaved under mild conditions. Organic and Biomolecular Chemistry, 17(8), 2110-2115. https://doi.org/10.1039/c8ob02899k
- Gimenez, D., Zhou, G., Hurley, M. F., Aguilar, J. A., Voelz, V. A., & Cobb, S. L. (2019). Fluorinated Aromatic Monomers as Building Blocks To Control α-Peptoid Conformation and Structure. Journal of the American Chemical Society, 141(8), 3430-3434. https://doi.org/10.1021/jacs.8b13498
- Gimenez, D., Aguilar, J., Bromley, E., & Cobb, S. (2018). Stabilising Peptoid Helices Using Non‐Chiral Fluoroalkyl Monomers. Angewandte Chemie International Edition, 57(33), 10549-10553. https://doi.org/10.1002/anie.201804488
- Steer, A., Bolt, H., Brittain, W., & Cobb, S. (2018). A Direct Route for the Preparation of Fmoc/OtBu Protected Iodotyrosine. Tetrahedron Letters, 59(27), 2644-2646. https://doi.org/10.1016/j.tetlet.2018.05.061
- Bolt, H., Kleijn, L., Martin, N., & Cobb, S. (2018). Synthesis of Antibacterial Nisin–Peptoid Hybrids Using Click Methodology. Molecules, 23(7), Article 1566. https://doi.org/10.3390/molecules23071566
- Grigolato, L., Brittain, W., Hudson, A., Czyzewska, M., & Cobb, S. (2018). Synthesis of pentafluorosulfanyl (SF5) containing aromatic amino acids. Journal of Fluorine Chemistry, 212, 166-170. https://doi.org/10.1016/j.jfluchem.2018.06.006
- Webster, A., & Cobb, S. (2018). Recent Advances in the Synthesis of Peptoid Macrocycles. Chemistry - A European Journal, 24(30), 7560-7573. https://doi.org/10.1002/chem.201705340
- Bolt, H. L., Eggimann, G. A., Jahoda, C. A., Zuckermann, R. N., Sharples, G. J., & Cobb, S. L. (2017). Exploring the links between peptoid antibacterial activity and toxicity. MedChemComm, 8(5), 886-896. https://doi.org/10.1039/c6md00648e
- Bolt, H., Williams, C., Brooks, R., Zuckermann, R., Cobb, S., & Bromley, E. (2017). Log D versus HPLC derived hydrophobicity: The development of predictive tools to aid in the rational design of bioactive peptoids. Biopolymers, 108(4), Article e23014. https://doi.org/10.1002/bip.23014
- Bolt, H., Denny, P., & Cobb, S. (2016). An Efficient Method for the Synthesis of Peptoids with Mixed Lysine-type/Arginine-type Monomers and Evaluation of Their Anti-leishmanial Activity. Journal of Visualized Experiments, 117, Article e54750. https://doi.org/10.3791/54750
- Bolt, H., Eggimann, G., Denny, P., & Cobb, S. (2016). Enlarging the chemical space of anti-leishmanials: a structure-activity relationship study of peptoids against Leishmania mexicana, a causative agent of cutaneous leishmaniasis. MedChemComm, 7(5), 799-805. https://doi.org/10.1039/c6md00060f
- Eggimann, G., Sweeney, K., Bolt, H., Rozatian, N., Cobb, S., & Denny, P. (2015). The Role of Phosphoglycans in the Susceptibility of Leishmania mexicana to the Temporin Family of Anti-Microbial Peptides. Molecules, 20(2), 2775-2785. https://doi.org/10.3390/molecules20022775
- Eggimann, G., Bolt, H., Denny, P., & Cobb, S. (2015). Investigating the Anti-leishmanial Effects of Linear Peptoids. ChemMedChem, 10(3), 233-237. https://doi.org/10.1002/cmdc.201402416
- Webster, A. M., Coxon, C. R., Kenwright, A. M., Sandford, G., & Cobb, S. L. (2014). A mild method for the synthesis of a novel dehydrobutyrine-containing amino acid. Tetrahedron, 70(31), 4661-4667. https://doi.org/10.1016/j.tet.2014.05.031
- Foster, J., Edkins, R., Cameron, G., Colgin, N., Fucke, K., Ridgeway, S., …Steed, J. (2014). Blending gelators to tune gel properties and probe anion-induced disassembly. Chemistry - A European Journal, 20(1), 279-291. https://doi.org/10.1002/chem.201303153
- West, C., Hudson, A., Cobb, S., & Verlet, J. (2013). Communication: Autodetachment versus internal conversion from the S1 state of the isolated GFP chromophore anion. The Journal of Chemical Physics, 139(7), https://doi.org/10.1063/1.4819078
- Trmčić, M., Chadbourne, F., Brear, P., Denny, P., Cobb, S., & Hodgson, D. (2013). Aqueous synthesis of N,S,-dialkylthiophosphoramidates: design, optimisation and application to library construction and antileishmanial testing. Organic and Biomolecular Chemistry, 11(16), 2660-2675. https://doi.org/10.1039/c3ob27448a
- Colgin, N., Tatum, N., Pohl, E., Cobb, S., & Sandford, G. (2012). Synthesis and molecular structure of a perfluorinated pyridyl carbanion. Journal of Fluorine Chemistry, 133, 33-37. https://doi.org/10.1016/j.jfluchem.2011.09.013
- Chadbourne, F., Raleigh, C., Ali, H., Denny, P., & Cobb, S. (2011). Studies on the antileishmanial properties of the antimicrobial peptides temporin A, B and 1Sa. Journal of Peptide Science, 17(11), 751-755. https://doi.org/10.1002/psc.1398
- Cobb, S., & Denny, P. (2010). Antimicrobial peptides for leishmaniasis. Current opinion in investigational drugs, 11(8), 868-875

