Staff profile
Professor David Hodgson
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42123 |
Biography
Research Overview
My research group employs the mechanistic tools of physical organic chemistry to approach biological and synthetic chemistry problems. Key themes are the use of kinetics and the understanding and exploitation of physicochemical properties to enable more effective synthetic chemistry and bioconjugation procedures.
We use these ideas to deliver new methods for the preparation of nucleosides and nucleotides. Our ability to understand reactivity, especially in aqueous solutions, has enabled us to develop convenient bioconjugation procedures for nucleic acids. Our mechanistic approach is also applied to enzymes and understanding polysaccharide biopolymer systems.
Nucleosides and Nucleotides
We use aqueous and non-aqueous approaches to prepare nucleosides, nucleotides and phosphate ester mimics. Nucleosides are challenging substrates for synthetic chemists because of their solubility characteristics. They are often barely soluble in both organic solvents and water as a result of intermolecular interactions between nucleoside molecules. This problem is particularly acute for guanosine systems that form gels through hydrogen bonding interactions between guanine base units. We have overcome this problem through using basic conditions that remove a key hydrogen bond donor owing to its relatively low pKa value (~9.5). Once the proton has been removed, substrates are soluble in aqueous media, however, this mandates the use of other tactics to allow functional group transformations to take place and subsequent purification of crude materials to be undertaken.
When organic solvents are employed, there is a requirement to use organic-solvent-soluble phosphate anions to prepare nucleoside di- and triphosphate species. Traditionally, alkylammonium salts have been used in this role, however, they are extremely hygroscopic and the water that they introduce can often have hugely deleterious effects on methods that are used to form phosphoanhydride bonds. We have prepared an alternative organic cation-pyrophosphate salt that does not take up moisture and is a convenient replacement for alkylammoniums.
Understanding Reactivity through Kinetics
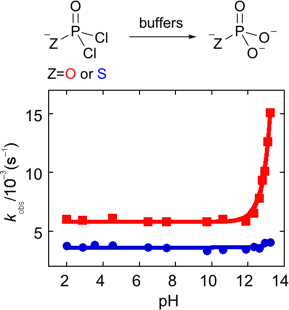
We use kinetics to understand how molecules react with each other and how we can improve selectivity through understaning the relative rates of competing hydrolysis processes. Phosphorus (V) chlorides are often considered to be incompatioble with water, however, through understaning the relative rates of hydrolysis vs aminolysis processes we have developed 'click' processes that use POCl3, PSCl3 and their hydrolysis products, which are also effective phosphorylating agents.
We have found that phosphodichloridate anion and its thio analogue, which are both water soluble, are highly selective N-phosphoryaltion agents. Their selectivity derives from the fact that they are anionic AND electrophilic, thus they discriminate against hydroxide anion as a competing nucleophile, but not against neutral amines. Their half-lives are in the order of minutes making them convenient to use without needing rapid mixing systems.
Bioconjugates
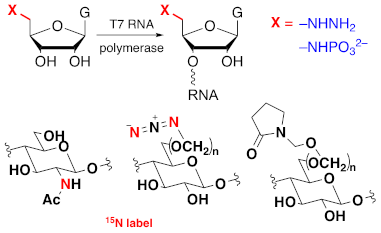
We have developed suites of bioconjugation protocols for nucleic acid and polysaccharide biopolymer systems. Our nucleic acid studies build on our synthetic protocols that give ready access to guanosine derivatives that are initiators for T7 RNA polymerase. The 'handles' that are incorporated can be readily modified with affinity tags or fluorphores for immobilisation and detection purposes.
Polysaccharide biopolymers are the most abundant resources offered by nature and we can capitalise upon them through the development of new conjugation techniques that provide modified systems with new materials properties and bioactivities.
Research interests
- Enzymes
- Nucleotides
- Physical Organic Chemistry
- Aqueous Reactivity
- Nucleic Acids
- Bioconjugation
Publications
Chapter in book
Journal Article
- Hampton, A. S., Hodgson, D. R. W., McDougald, G., Wang, L., & Sandford, G. (2024). Synthesis of 2,2-difluoro-1,3-diketone and 2,2-difluoro-1,3-ketoester derivatives using fluorine gas. Beilstein Journal of Organic Chemistry, 20, 460-469. https://doi.org/10.3762/bjoc.20.41
- Murray, J., Hodgson, D. R., & O’Donoghue, A. C. (2023). Going Full Circle with Organocatalysis and Biocatalysis: The Latent Potential of Cofactor Mimics in Asymmetric Synthesis. Journal of Organic Chemistry, https://doi.org/10.1021/acs.joc.2c02747
- Guillen-Garcia, A., Gibson, S., Jordan, C., Ramaswamy, V., Linthwaite, V., Bromley, E., …Cann, M. (2022). Allophycocyanin A is a carbon dioxide receptor in the cyanobacterial phycobilisome. Nature Communications, 13, Article 5289. https://doi.org/10.1038/s41467-022-32925-6
- Linthwaite, V., Pawloski, W., Pegg, H., Townsend, P., Thomas, M., Brown, A., …Cann, M. (2021). Ubiquitin is a carbon dioxide-binding protein. Science Advances, 7(39), Article eabi5507. https://doi.org/10.1126/sciadv.abi5507
- Rozatian, N., & Hodgson, D. R. (2021). Reactivities of electrophilic N–F fluorinating reagents. Chemical Communications, 57(6), 683-712. https://doi.org/10.1039/d0cc06339h
- Quinn, P., Smith, M. S., Zhu, J., Hodgson, D. R., & O’Donoghue, A. C. (2021). Triazolium Salt Organocatalysis: Mechanistic Evaluation of Unusual Ortho-Substituent Effects on Deprotonation. Catalysts, 11(9), Article 1055. https://doi.org/10.3390/catal11091055
- Rozatian, N., Harsanyi, A., Murray, B. J., Hampton, A. S., Chin, E. J., Cook, A. S., …Sandford, G. (2020). Kinetics of electrophilic fluorination of steroids and epimerisation of fluorosteroids. Chemistry - A European Journal, 26(52), 12027-12035. https://doi.org/10.1002/chem.202001120
- Rozatian, N., Beeby, A., Ashworth, I. W., Sandford, G., & Hodgson, D. R. (2019). Enolization rates control mono- versus di-fluorination of 1,3-dicarbonyl derivatives. Chemical Science, 10(44), 10318-10330. https://doi.org/10.1039/c9sc04185k
- Bose, S., & Hodgson, D. R. (2019). Stereoselective Syntheses of 3’-Hydroxyamino- and 3’-Methoxyamino-2’,3’-Dideoxynucleosides. Organic Letters, 21(22), 9084-9088. https://doi.org/10.1021/acs.orglett.9b03474
- Rozatian, N., Ashworth, I. W., Sandford, G., & Hodgson, D. R. (2018). A Quantitative Reactivity Scale for Electrophilic Fluorinating Reagents. Chemical Science, 9(46), 8692-8702. https://doi.org/10.1039/c8sc03596b
- Linthwaite, V., Janus, J., Brown, A., Wong-Pascua, D., O’Donoghue, A., Porter, A., …Cann, M. (2018). The identification of carbon dioxide mediated protein post-translational modifications. Nature Communications, 9, Article 3092. https://doi.org/10.1038/s41467-018-05475-z
- Eguaogie, O., Conlon, P. F., Ravalico, F., Sweet, J. S., Elder, T. B., Conway, L. P., …Vyle, J. S. (2017). Nucleophilic displacement reactions of 5′-derivatised nucleosides in a vibration ball mill. Beilstein Journal of Organic Chemistry, 13, 87-92. https://doi.org/10.3762/bjoc.13.11
- Conway, L., Mikkola, S., O’Donoghue, A., & Hodgson, D. (2016). The Synthesis, Conformation and Hydrolytic Stability of an N,S-bridging Thiophosphoramidate Analogue of Thymidylyl-3ʹ,5ʹ-Thymidine. Organic and Biomolecular Chemistry, 14(30), 7361-7367. https://doi.org/10.1039/c6ob01270a
- Eguaogie, O., Cooke, L. A., Martin, P. M., Ravalico, F., Conway, L. P., Hodgson, D. R., …Vyle, J. S. (2016). Synthesis of novel pyrophosphorothiolate-linked dinucleoside cap analogues in a ball mill. Organic and Biomolecular Chemistry, 14(4), 1201-1205. https://doi.org/10.1039/c5ob02061a
- Joubert, F., Yeo, R. P., Sharples, G. J., Musa, O. M., Hodgson, D. R., & Cameron, N. R. (2015). Preparation of an antibacterial poly(ionic liquid) graft copolymer of hydroxyethyl cellulose. Biomacromolecules, 16(12), 397-3979. https://doi.org/10.1021/acs.biomac.5b01300
- Dano, M., Elmeranta, M., Hodgson, D. R., Jaakkola, J., Korhonen, H., & Mikkola, S. (2015). Metal–ion promoted cleavage of nucleoside diphosphosugars: A model for reactions of phosphodiester bonds in carbohydrates. JBIC Journal of Biological Inorganic Chemistry, 20(8), 1299-1306. https://doi.org/10.1007/s00775-015-1308-9
- Townsend, P., Rodgers, T., Glover, L., Korhonen, H., Richards, S., Colwell, L., …Cann, M. (2015). The role of protein-ligand contacts in allosteric regulation of the Escherichia coli Catabolite Activator Protein. Journal of Biological Chemistry, 290(36), 22225-22235. https://doi.org/10.1074/jbc.m115.669267
- Korhonen, H. J., Bolt, H. L., Vicente-Gines, L., Perks, D. C., & Hodgson, D. R. (2015). PPN pyrophosphate: A New Reagent for the Preparation of Nucleoside Triphosphates. Phosphorus, Sulfur, and Silicon and the Related Elements, 190(5-6), 758-762. https://doi.org/10.1080/10426507.2014.984032
- Carvalho, A., O’Donoghue, A., Hodgson, D., & Kamerlin, S. (2015). Understanding Thio-Effects in Simple Phosphoryl Systems: Role of Solvent Effects and Nucleophile Charge. Organic and Biomolecular Chemistry, 13(19), 5391-5398. https://doi.org/10.1039/c5ob00309a
- Korhonen, H., Bolt, H., & Hodgson, D. (2015). A procedure for the preparation and isolation of nucleoside-5’-diphosphates. Beilstein Journal of Organic Chemistry, 11, 469-472. https://doi.org/10.3762/bjoc.11.52
- Joubert, F., Sharples, G. J., Musa, O. M., Hodgson, D. R., & Cameron, N. R. (2015). Preparation, properties, and antibacterial behavior of a novel cellulose derivative containing lactam groups. Journal of Polymer Science Part A: Polymer Chemistry, 53(1), 68-78. https://doi.org/10.1002/pola.27441
- Joubert, F., Musa, O. M., Hodgson, D. R., & Cameron, N. R. (2014). The preparation of graft copolymers of cellulose and cellulose derivatives using ATRP under homogeneous reaction conditions. Chemical Society Reviews, 43(20), 7217-7235. https://doi.org/10.1039/c4cs00053f
- Conway, L., Delley, R., Neville, J., Freeman, G., Maple, H., Chan, V., …Hodgson, D. (2014). The Aqueous N-Phosphorylation and N-Thiophosphorylation of Aminonucleosides. RSC Advances, 2014(73), 38663-38671. https://doi.org/10.1039/c4ra08317b
- Korhonen, H. J., Conway, L. P., & Hodgson, D. R. (2014). Phosphate analogues in the dissection of mechanism. Current Opinion in Chemical Biology, 21, 63-72. https://doi.org/10.1016/j.cbpa.2014.05.001
- Joubert, F., Musa, O., Hodgson, D. R., & Cameron, N. R. (2014). Graft copolymers of hydroxyethyl cellulose by a ‘grafting to’ method: 15N labelling as a powerful characterisation tool in ‘click’ polymer chemistry. Polymer Chemistry, 6(9), 1567-1575. https://doi.org/10.1039/c4py01413h
- Skipsey, M., Hack, G., Hooper, T., Shankey, M., Conway, L., Schröder, M., & Hodgson, D. (2013). 5’-Deoxy-5’-hydrazinylguanosine as an initiator of T7 RNA polymerase-catalyzed transcriptions for the preparation of labeling-ready RNAs. Nucleosides, Nucleotides and Nucleic Acids, 32(12), 670-681. https://doi.org/10.1080/15257770.2013.851393
- Trmčić, M., Chadbourne, F., Brear, P., Denny, P., Cobb, S., & Hodgson, D. (2013). Aqueous synthesis of N,S,-dialkylthiophosphoramidates: design, optimisation and application to library construction and antileishmanial testing. Organic and Biomolecular Chemistry, 11(16), 2660-2675. https://doi.org/10.1039/c3ob27448a
- Delley, R., Bandyopadhyay, S., Fox, M., Schliehe, C., Hodgson, D., Hollfelder, F., …O'Donoghue, A. (2012). peri-Dimethylamino substituent effects on proton transfer at carbon in α-naphthylacetate esters: a model for mandelate racemase. Organic and Biomolecular Chemistry, 10(3), 590-596. https://doi.org/10.1039/c1ob06525d
- Delley, R., O'Donoghue, A., & Hodgson, D. (2012). Hydrolysis studies of phosphodichloridate and thiophosphodichloridate ions. Journal of Organic Chemistry, 77(13), 5829-5831. https://doi.org/10.1021/jo300808m
- Trmčić, M., & Hodgson, D. (2011). Synthesis of thiophosphoramidates in water: Click chemistry for phosphates. Chemical Communications, 47(21), 6156-6158. https://doi.org/10.1039/c1cc11586c
- Norcliffe, J., Conway, L., & Hodgson, D. (2011). Reduction of alkyl and aryl azides with sodium thiophosphate in aqueous solutions. Tetrahedron Letters, 52(21), 2730-2732. https://doi.org/10.1016/j.tetlet.2011.03.083
- Hodgson, D., & Schröder, M. (2011). Chemical approaches towards unravelling kinase-mediated signalling pathways. Chemical Society Reviews, 40(3), 1211-1223. https://doi.org/10.1039/c0cs00020e
- Kwan, I., Delley, R., Hodgson, D., & Wu, G. (2011). Single atom modification leads to enhanced nucleotide self-assembly: the role of cation bridging. Chemical Communications, 47(13), 3882-3884. https://doi.org/10.1039/c0cc05654e
- Trmčić, M., & Hodgson, D. (2010). Kinetic studies and predictions on the hydrolysis and aminolysis of esters of 2-S-phosphorylacetates. Beilstein Journal of Organic Chemistry, 6, 732-741. https://doi.org/10.3762/bjoc.6.87
- Kirby, A., Davies, J., Fox, D., Hodgson, D., Goeta, A., Lima, M., …Nome, F. (2010). Ammonia oxide makes up some 20% of an aqueous solution of hydroxylamine. Chemical Communications, 46(8), 1302-1304. https://doi.org/10.1039/b923742a
- Watson, H., Apperley, D., Dixon, D., Edwards, R., & Hodgson, D. (2009). An Efficient Method for ¹⁵N-Labeling of Chitin in Fungi. Biomacromolecules, 10(4), 793-797. https://doi.org/10.1021/bm8012814
- Townsend, P., Holliday, P., Fenyk, S., Hess, K., Gray, M., Hodgson, D., & Cann, M. (2009). Stimulation of Mammalian G-protein-responsive Adenylyl Cyclases by Carbon Dioxide. Journal of Biological Chemistry, 284(2), 784-791. https://doi.org/10.1074/jbc.m807239200
- Brear, P., Freeman, G., Shankey, M., Trmčić, M., & Hodgson, D. (2009). Aqueous methods for the preparation of 5′-substituted guanosine derivatives. Chemical Communications, 2009(33), 4980-4981. https://doi.org/10.1039/b908727c
- Brazier-Hicks, M., Evans, K., Cunningham, O., Hodgson, D., Steel, P., & Edwards, R. (2008). Catabolism of glutathione conjugates in Arabidopsis thaliana: role in metabolic reactivation of the herbicide safener fenclorim. Journal of Biological Chemistry, 283(30), 21102-21112. https://doi.org/10.1074/jbc.m801998200
- Williamson, D., & Hodgson, D. R. (2008). Preparation and purification of 5 '-amino-5 '-deoxyguanosine-5 '-N-phosphoramidate and its initiation properties with T7 RNA polymerase. Organic and Biomolecular Chemistry, 6(6), 1056-1062. https://doi.org/10.1039/b717896d
- Williamson, D., Cann, M., & Hodgson, D. (2007). Synthesis of 5'-Amino-5'-deoxyguanosine-5'-N-phosphoramidate and its Enzymatic Incorporation at the 5'-Termini of RNA Molecules. Chemical Communications, 2007(47), 5096-5098. https://doi.org/10.1039/b712066d
- Hammer, A., Hodgson, D., & Cann, M. (2006). Regulation of prokaryotic adenylyl cyclases by CO₂. Biochemical Journal, 396(2), 215-218. https://doi.org/10.1042/bj20060372
- Asaad, N., Davies, J., Hodgson, D., Kirby, A., van Vliet, L., & Ottavi, L. (2005). The search for efficient intramolecular proton transfer from carbon: the kinetically silent intramolecular general base-catalysed elimination reaction of O-phenyl 8-dimethylamino-1-naphthaldoximes. Journal of Physical Organic Chemistry, 18(2), 101-109. https://doi.org/10.1002/poc.858
- Hodgson, D., & Sanderson, J. (2004). The Synthesis of Peptides and Proteins Containing Non-Natural Amino Acids. Chemical Society Reviews, 33(7), 422-430. https://doi.org/10.1039/b312953p
- Hodgson, D., & Suga, H. (2004). Mechanistic studies on acyl-transferase ribozymes and beyond. Biopolymers, 73(1), 130-150. https://doi.org/10.1002/bip.10518
- Davis, B., Sala, R., Hodgson, D., Ullman, A., Khumtaveeporn, K., Estell, D., …Jones, J. (2003). Selective protein degradation by ligand-targeted enzymes: towards the creation of catalytic antagonists. ChemBioChem, 4(6), 533-537. https://doi.org/10.1002/cbic.200300591
- Bessho, Y., Hodgson, D., & Suga, H. (2002). A tRNA aminoacylation system for non-natural amino acids based on a programmable ribozyme. Nature Biotechnology, 20(7), 723-728. https://doi.org/10.1038/nbt0702-723
- Hartwell, E., Hodgson, D., & Kirby, A. (2000). Exploring the limits of efficiency of proton-transfer catalysis in models and enzymes. Journal of the American Chemical Society, 122(38), 9326-9327. https://doi.org/10.1021/ja002335m
- Hodgson, D., Kirby, A., & Feeder, N. (1999). The case of the missing acetylene. The mechanism of an intramolecular S-N(V) reaction and a new route to 1-methylbenzo[de]quinolines. Journal of the Chemical Society. Perkin transactions 1, 949-954. https://doi.org/10.1039/a900090i
- GenreGrandpierre, A., Tellier, C., Loirat, M., Blanchard, D., Hodgson, D., Hollfelder, F., & Kirby, A. (1997). Catalysis of the Kemp elimination by antibodies elicited against a cationic hapten. Bioorganic and Medicinal Chemistry Letters, 7(19), 2497-2502. https://doi.org/10.1016/s0960-894x%2897%2910003-8
- Colbert, M., Hodgson, D., Lewis, J., Raithby, P., & Long, N. (1995). Synthesis, Characterization and Electrochemical Studies of 1',6'-Bis(Ethynyl)Biferrocene and Some Metal-Complexes - Novel Heterometallic Compounds Towards Nonlinear Optics. Polyhedron, 14(19), 2759-2766. https://doi.org/10.1016/0277-5387%2895%2900143-g
Patent

