Staff profile
Professor Ivana Evans
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42594 |
| Member of the Institute of Medieval and Early Modern Studies |
Biography
Ivana Radosavljević Evans was born in Belgrade, the capital of Serbia. She obtained her first degree in Physical Chemistry at the University of Belgrade and her PhD in Chemistry at Oregon State University, under the supervision of Professor Art Sleight, in 1999. After moving to the UK, she joined the group of Professor Judith Howard at the Durham University Department of Chemistry as a post doctoral research associate. She was the recipient of the Cambridge Crystallographic Data Centre Prize for Younger Scientist Award for 2003. In 2005, she took up an independent RCUK Academic Fellowship. She was appointed a Lecturer in in Structural/Materials Chemistry in 2009, promoted to Senior Lecturer in 2011, Reader in 2015 and Professor in 2019.
Ivana was the Chair (2011 - 2014) of the Physical Crystallography Group of the British Crystallographic Association and the Structural Condensed Matter Physics Group of the Institute of Physics. She has served on the Diffraction peer review panel for Diamond Light Source (2010-2014) and on the ISIS Diffraction peer review panel (2015-2017), and is currently a member of the RSC Materials Chemistry Division Council, the RSC Faraday Discussions Standing Committee and the IUCr Commission on Powder Diffraction.
Research interests
My research interests are in the area of solid state chemistry and structure-property relationships of functional materials across the chemical spectrum. Properties of materials and hence their practical applications are intimately linked to their three-dimensional structures. By understanding the structures, we aim to understand the behaviour of materials and feed this knowledge back into our synthetic efforts, with the ultimate aim of making new functional materials with improved properties and prospects for practical applications.
Current research projects are focussed on three main areas: development of new oxide ion conductors, hydrogen-bonded organic functional materials and polymorphism. These projects encompass synthetic work (solid state, solvothermal, crystal growth), characterisation of static and dynamic structure (powder and single crystal X-ray and neutron diffraction, electron microscopy, total scattering, solid state NMR, inelastic/quasi-elastic neutron scattering, impedance spectroscopy) and computational modelling (ab-initio molecular dynamics, AIMD).
New oxide ion conductors
Oxide ion conductors are technologically important materials, essential for applications such as oxygen sensors and pumps, ceramic membranes for oxygen separation and partial oxidation of light hydrocarbons and solid oxide fuel cells (SOFCs), where they act as electrolytes transporting O2- ions to react with a fuel such as hydrogen in the direct clean conversion of chemical to electrical energy. Our current research focusses on the discovery of new materials with high ionic conductivity, particularly at at lower temperatures (500-750oC)1-5. Ionic mobility in solids is of fundamental significance; however, if suitable materials are found, the SOFC operating temperatures could be lowered, thus alleviating the two main problems (reliability and cost) which currently limit their widerspread use in energy generation.
We recently reported the remarkably high low-temperature ionic conductivity in Bi1-xVxO1.5+x (x = 0.087, 0.095; s~3.9 ×10-2 S/cm at 500oC) and the roles of the different structural building blocks in this process.1 We have attributed this remarkable behaviour to the simultaneous presence of three key factors: a highly polarisable sublattice with vacancies, central atoms able to support variable coordination numbers and geometries, and the rotational flexibility of the these coordination polyhedra. Importantly, the structure is a stable, pseudo-cubic 3 × 3 × 3 fluorite-based superstructure.

Figure 1: Oxide ion migration pathways in Bi1-xVxO1.5+x obtained by AIMD simulations; white displacement clouds represent space visited by oxide ions; V coordination polyhedra VOx shown in red; OBi4 groups and Bi atoms shown in yellow. (a) direct O2- exchange between VOx groups; (b) O2- exchange between VOx groups via a OBi4 tetrahedron; (c) O2- vacancy-hopping through the Bi-O subllatice.
Functional hydrogenous materials and short strong hydrogen bonds
Behaviour of protons in short strong hydrogen bonds (SSHB), including migration and ordering, has implications for charge and energy transfer in chemical and biological systems in the solid state, including ferroelectric and non-linear optical materials, magnetic coordination polymers and enzyme reactions. The aim of this project is to investigate solid-state structure and dynamics of SSHB systems with proton migration and gain insight into the specific structural features and phonon modes that drive this behaviour. Synthesis, crystallisation and variable temperature X-ray diffraction work are carried out at Durham; neutron diffraction, inelastic neutron scattering and computational modelling at the ILL in Grenoble, France.
Temperature-induced proton migration has traditionally been associated with the hydrogen bond length. Our recent experimental and computational work on the four isotopologues of 3,5-pyridinedicarboxylic acid demonstrate that a local picture of the H/D environment is insufficient to understand the migration and transfer phenomena, even in simple molecular systems. Deuterated 3,5-pyridinedicarboxylic acid exhibits deuteron migration of a magnitude unprecedented in this class of materials. Importantly, deuteron migration is significantly more pronounced (0.32 Å vs. 0.09 Å) than the proton migration. Our results provide significant new insight into how an isosymmetric phase transition and vibrational free-energy stabilisation drive this behaviour and computationally predict the isotope effect observed experimentally.6
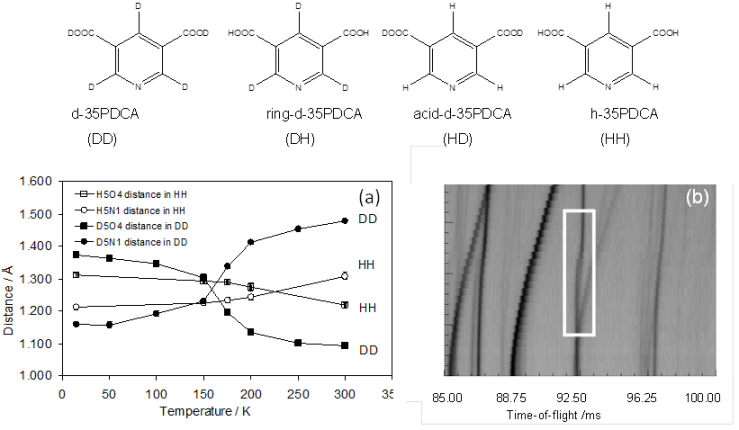
Figure 2: Isotopologues of the 3,5-pyridinedicarboxylic acid. (a) temperature dependence of the D5-O4 and D5-N1 distances in d-35PDCA and H5-O4 and H5-N1 in h-35PDCA obtained from single-crystal neutron diffraction; (b) variable-temperature powder neutron diffraction reveals a first-order phase transition associated with remarkably large deuteron migration in d-35PDCA.
Polymorphism of pharmaceutical solids
Increasingly stringent and demanding regulatory expectations imposed on the pharmaceutical industry necessitate a full, accurate and precise characterisation of its products. This is particularly important for different polymorphs of drug molecules, since they can differ in properties which determine therapeutic performance, such as dissolution rates and bioavailability.
This project involves identification and determination of crystal structures of polymorphic pharmaceutically relevant materials, studies of thermal stability and thermodynamic relationships between polymorphs, as well as processing-induced effects (e.g. by grinding, pressing, freeze drying).8-9

Figure 3: Concomitant polymorphism in chincomeronic acid (3,4-pyridinedicarboxylic acid), commonly used as a multifunctional ligand in coordination chemistry: molecular structures of Form I (needles) and Form II (blocks) differ only in the position of one hydrogen atom, leading to different crystal structure and hydrogen bonding motifs in the solid state.
Applications of powder diffraction in archaeology and art conservation
Applications of modern analytical techniques to archaeological and art objects fall into two major areas. Archaeometric studies focus on various provenance-related issues. Conservation science deals with questions related to degradation of objects with time or under certain environmental conditions. Powder X-Ray diffraction is a valuable tool in both of these areas. It can give information about the mineralogical composition, pigments used for decoration and degradation products, thus shedding light on geographical origin, manufacture processes and period, as well as preventative or remedial conservation measures.
In these projects, we collaborate with archaeologists and conservation scientists in the UK and abroad. Recent work includes Byzantine ceramics provenancing10, (Fig. 4, in collaboration with Dr. Ljiljana Damjanovic, Faculty of Physical Chemistry, University of Belgrade, Serbia) and identification of corrosion products from an ancient Egyptian figurine (Fig. 5, in collaboration with Hannah Urquhart and Dr. Chris Caple, Department of Archaeology, Durham University).

Research interests
- Oxide Ion Conductors
- Solid State Chemistry
- Functional materials
Publications
Journal Article
- Schwaighofer, B., Appel, M., Gonzalez, M. A., & Radosavljevic Evans, I. (2024). Oxide ion dynamics in hexagonal perovskite mixed conductor Ba 7 Nb 4 MoO 20: a comprehensive ab initio molecular dynamics study. Materials Advances, https://doi.org/10.1039/d3ma00955f
- Schwaighofer, B., Gonzalez, M. A., Appel, M., Koza, M. M., & Evans, I. R. (2023). Oxide Ion Mobility in V- and P-doped Bi2O3-Based Solid Electrolytes: Combining Quasielastic Neutron Scattering with Ab Initio Molecular Dynamics. Chemistry of Materials, 35(3), 1125-1133. https://doi.org/10.1021/acs.chemmater.2c03103
- Mullins, A. L., Ćirić, A., Zekovic, I., Williams, J. G., Dramicanin, M., & Evans, I. R. (2022). Dual-emission luminescence thermometry using LaGaO3:Cr3+, Nd3+ phosphors. Journal of Materials Chemistry C Materials for optical and electronic devices, 10(28), 10396-10403. https://doi.org/10.1039/d2tc02011d
- Mullins, A. L., Ćirić, A., Ristić, Z., Williams, J. G., Radosavljević Evans, I., & Dramićanin, M. D. (2022). Double-deconvolution method for the separation of thermalised emissions from chromium-doped lanthanum gallate and its potential in luminescence-based thermometry. Journal of Luminescence, 246, https://doi.org/10.1016/j.jlumin.2022.118847
- Fuller, C. A., Murrell, J. I., Blom, D. A., Vogt, T., Zhang, W., Halasyamani, P. S., …Evans, J. S. (2022). Oxide ion conductivity, proton conductivity and phase transitions in perovskite-derived Ba3–xSrxYGa2O7.5 0 x 3 materials. Chemistry of Materials, 34(7), 3185-3196. https://doi.org/10.1021/acs.chemmater.1c04372
- Fuller, C. A., Blom, D. A., Vogt, T., Radosavljevic Evans, I., & Evans, J. S. (2022). Oxide Ion and Proton Conductivity in a Family of Highly Oxygen-Deficient Perovskite Derivatives. Journal of the American Chemical Society, 144(1), 615-624. https://doi.org/10.1021/jacs.1c11966
- Fuller, C. A., Gutmann, M. J., Ling, C. D., Wang, C., Zhang, W., Halasyamani, P. S., …Evans, J. S. (2022). Defects and disorder in apatite-type silicate oxide ion conductors: implications for conductivity. Journal of Materials Chemistry A: materials for energy and sustainability, 10(27), https://doi.org/10.1039/d2ta02328h
- Dramićanin, M. D., Marciniak, Ł., Kuzman, S., Piotrowski, W., Ristić, Z., Periša, J., …Ma, C. (2022). Mn5+-activated Ca6Ba(PO4)4O near-infrared phosphor and its application in luminescence thermometry. Light: Science and Applications, 11, Article 279. https://doi.org/10.1038/s41377-022-00958-7
- Rodriguez-Garcia, M. M., Ćirić, A., Ristić, Z., Williams, J. G., Dramicanin, M., & Evans, I. R. (2021). Narrow-band red phosphors of high colour purity based on Eu3+-activated apatite-type Gd9.33(SiO4)6O2. Journal of Materials Chemistry C Materials for optical and electronic devices, 9(23), 7474-7484. https://doi.org/10.1039/d1tc01330k
- Auckett, J. E., Lopez-Odriozola, L., Clark, S. J., & Radosavljevic Evans, I. (2021). Exploring the nature of the fergusonite–scheelite phase transition and ionic conductivity enhancement by Mo6+ doping in LaNbO4. Journal of Materials Chemistry A: materials for energy and sustainability, 9(7), 4091-4102. https://doi.org/10.1039/d0ta07453e
- Evans, J. S., & Radosavljevic Evans, I. (2021). Structure Analysis from Powder Diffraction Data: Rietveld Refinement in Excel. Journal of Chemical Education, 98(2), 495-505. https://doi.org/10.1021/acs.jchemed.0c01016
- Rahim, W., Skelton, J. M., Savory, C. N., Evans, I. R., Evans, J. S., Walsh, A., & Scanlon, D. O. (2020). Polymorph exploration of bismuth stannate using first-principles phonon mode mapping. Chemical Science, 11(30), 7904-7909. https://doi.org/10.1039/d0sc02995e
- Fuller, C. A., Berrod, Q., Frick, B., Johnson, M. R., Avdeev, M., Evans, J. S., & Evans, I. R. (2020). Oxide Ion and Proton Conductivity in Highly Oxygen-Deficient Cubic Perovskite SrSc0.3Zn0.2Ga0.5O2.4. Chemistry of Materials, 32(10), 4347-4357. https://doi.org/10.1021/acs.chemmater.0c01378
- Chambers, M. S., McCombie, K. S., Auckett, J. E., McLaughlin, A. C., Irvine, J. T., Chater, P. A., …Evans, I. R. (2019). Hexagonal perovskite related oxide ion conductor Ba3NbMoO8.5: phase transition, temperature evolution of the local structure and properties. Journal of Materials Chemistry A: materials for energy and sustainability, 7(44), 25503-25510. https://doi.org/10.1039/c9ta08378b
- Chambers, M. S., Chater, P. A., Evans, I. R., & Evans, J. S. (2019). Average and Local Structure of Apatite-Type Germanates and Implications for Oxide Ion Conductivity. Inorganic Chemistry, 58(21), 14853-14862. https://doi.org/10.1021/acs.inorgchem.9b02544
- Fuller, C. A., Berrod, Q., Frick, B., Johnson, M. R., Clark, S. J., Evans, J. S., & Evans, I. R. (2019). Brownmillerite-type Sr2ScGaO5 Oxide Ion Conductor: Local Structure, Phase Transition and Dynamics. Chemistry of Materials, 31(18), 7395-7404. https://doi.org/10.1021/acs.chemmater.9b02051
- Rodriguez-Garcia, M. M., Williams, J. G., & Evans, I. R. (2019). Single-Phase White-Emitting Phosphors Based on Apatite-Type Gadolinium Silicate, Gd9.33(SiO4)6O2 Doped with Dy3+, Eu3+ and Tb3+. Journal of Materials Chemistry C Materials for optical and electronic devices, 7(25), 7779-7787. https://doi.org/10.1039/c9tc02336d
- Peet, J. R., Fuller, C. A., Frick, B., Koza, M. M., Johnson, M. R., Piovano, A., & Evans, I. R. (2019). Insight into Design of Improved Oxide Ion Conductors: Dynamics and Conduction Mechanisms in the Bi0.913V0.087O1.587 Solid Electrolyte. Journal of the American Chemical Society, 141(25), 9989-9997. https://doi.org/10.1021/jacs.9b03743
- Liu, H., Gutmann, M. J., Stokes, H. T., Campbell, B. J., Evans, I. R., & Evans, J. S. (2019). Super-colossal uniaxial negative thermal expansion in chloranilic acid pyrazine, CA-Pyz. Chemistry of Materials, 31(12), 4514-4523. https://doi.org/10.1021/acs.chemmater.9b01135
- Stojanović, S., Bikić, V., Miličić, L., Radosavljević Evans, I., Scarlett, N. V., Brand, H. E., & Damjanović-Vasilić, L. (2019). Evidence of continuous pottery production during the late Byzantine period in the Studenica Monastery, a UNESCO World Heritage Site. Microchemical Journal, 146, 557-567. https://doi.org/10.1016/j.microc.2019.01.056
- Dunstan, M. T., Halat, D. M., Tate, M. L., Evans, I. R., & Grey, C. P. (2019). Variable-temperature multinuclear solid-state NMR study of oxide ion dynamics in fluorite-type bismuth vanadate and phosphate solid electrolytes. Chemistry of Materials, 31(5), 1704-1714. https://doi.org/10.1021/acs.chemmater.8b05143
- Auckett, J. E., Milton, K. L., & Evans, I. R. (2019). Cation distributions and anion disorder in Ba3NbMO8.5 (M = Mo, W) materials: Implications for oxide ion conductivity. Chemistry of Materials, 31(5), 1715-1719. https://doi.org/10.1021/acs.chemmater.8b05179
- Liu, H., Zhang, W., Halasyamani, P. S., Stokes, H. T., Campbell, B. J., Evans, J. S., & Evans, I. R. (2018). Understanding the Behavior of the Above-Room-Temperature Molecular Ferroelectric 5,6-Dichloro-2-methylbenzimidazole Using Symmetry Adapted Distortion Mode Analysis. Journal of the American Chemical Society, 140(41), 13441-13448. https://doi.org/10.1021/jacs.8b08591
- Gajić-Kvaščev, M., Bikić, V., Wright, V. J., Radosavljević Evans, I., & Damjanović-Vasilić, L. (2018). Archaeometric study of 17th/18th century painted pottery from the Belgrade Fortress. Journal of Cultural Heritage, 32, 9-21. https://doi.org/10.1016/j.culher.2018.01.018
- Peet, J. R., Chambers, M. S., Piovano, A., Johnson, M., & Evans, I. R. (2018). Dynamics in Bi(III)-containing Apatite-Type Oxide Ion Conductors: A Combined Computational and Experimental Study. Journal of Materials Chemistry A: materials for energy and sustainability, 6(12), 5129-5135. https://doi.org/10.1039/c8ta00546j
- Wind, J., Kayser, P., Zhang, Z., Radosavljevic Evans, I., & Ling, C. D. (2017). Stability and range of the type II Bi 1−x W x O 1.5+1.5x solid solution. Solid State Ionics, 308, 173-180. https://doi.org/10.1016/j.ssi.2017.07.015
- Tate, M., Fuller, C., Avdeev, M., Brand, H., McIntyre, G., & Radosavljevic Evans, I. (2017). Synthesis and characterisation of new Bi(iii)-containing apatite-type oxide ion conductors: the influence of lone pairs. Dalton Transactions, 46(37), 12494-12499. https://doi.org/10.1039/c7dt02871g
- Peet, J., Fuller, C., Frick, B., Zbiri, M., Piovano, A., Johnson, M., & Radosavljevic Evans, I. (2017). Direct Observation of Oxide Ion Dynamics in La2Mo2O9 on the Nanosecond Timescale. Chemistry of Materials, 29(7), 3020-3028. https://doi.org/10.1021/acs.chemmater.6b05507
- Tate, M. L., Blom, D. A., Avdeev, M., Brand, H. E., McIntyre, G. J., Vogt, T., & Evans, I. R. (2017). New Apatite-Type Oxide Ion Conductor, Bi2La8[(GeO4)6]O3: Structure, Properties, and Direct Imaging of Low-Level Interstitial Oxygen Atoms Using Aberration-Corrected Scanning Transmission Electron Microscopy. Advanced Functional Materials, 27(8), Article 1605625. https://doi.org/10.1002/adfm.201605625
- Lewis, J. W., Payne, J. L., Evans, I. R., Stokes, H. T., Campbell, B. J., & Evans, J. S. (2016). An Exhaustive Symmetry Approach to Structure Determination: Phase Transitions in Bi2Sn2O7. Journal of the American Chemical Society, 138(25), 8031-8042. https://doi.org/10.1021/jacs.6b04947
- Peet, J. R., Widdifield, C. M., Apperley, D. C., Hodgkinson, P., Johnson, M. R., & Evans, I. R. (2015). Na+ mobility in sodium strontium silicate fast ion conductors. Chemical Communications, 51(96), 17163-17165. https://doi.org/10.1039/c5cc06644a
- Kerr, H. E., Softley, L. K., Kuthuru, S., Nangia, A., Hodgkinson, P., & Evans, I. R. (2015). A furosemide – isonicotinamide cocrystal: An investigation of properties and extensive structural disorder. CrystEngComm, 17(35), 6707-6715. https://doi.org/10.1039/c5ce01183c
- Nunes, S. E., Wang, C., So, K., Evans, J. S., & Evans, I. R. (2015). Bismuth zinc vanadate, BiZn2VO6: New crystal structure type and electronic structure. Journal of Solid State Chemistry, 222, 12-17. https://doi.org/10.1016/j.jssc.2014.10.036
- Evans, I., Evans, J., Davies, H., Haworth, A., & Tate, M. (2014). On Sr1−xNaxSiO3−0.5x New Superior Fast Ion Conductors. Chemistry of Materials, 26(18), 5187-5189. https://doi.org/10.1021/cm502850m
- Harriss, B., Wilson, C., & Evans, I. R. (2014). Niclosamide Methanol Solvate and Niclosamide Hydrate: Structure, Solvent Inclusion Mode and Implications for Properties. Acta Crystallographica Section C: Structural Chemistry, 70(8), 758-763. https://doi.org/10.1107/s2053229614015496
- Frantsuzov, I., Ford, S., Radosavljevic Evans, I., Horsewill, A., Trommsdorff, H., & Johnson, M. (2014). Measurement of proton tunneling in short hydrogen bonds in single crystals of 3,5 pyridinedicarboxylic acid using nuclear magnetic resonance spectroscopy. Physical Review Letters, 113(1), Article 018301. https://doi.org/10.1103/physrevlett.113.018301
- Harriss, B. I., Vella-Zarb, L., Wilson, C., & Radosavljevic Evans, I. (2014). Furosemide Cocrystals: Structures, Hydrogen Bonding, and Implications for Properties. Crystal Growth and Design, 14(2), 783-791. https://doi.org/10.1021/cg401662d
- Payne, J., Tucker, M., & Evans, I. R. (2013). From Fluorite to Pyrochlore: Characterisation of Local and Average Structure of Neodymium Zirconate, Nd2Zr2O7. Journal of Solid State Chemistry, 205, 29-34. https://doi.org/10.1016/j.jssc.2013.07.001
- Payne, J., Farrell, J., Linsell, A., Johnson, M., & Evans, I. R. (2013). The Mechanism of Oxide Ion Conductivity in Bismuth Rhenium Oxide, Bi28Re2O49. Solid State Ionics, 244, 35-39. https://doi.org/10.1016/j.ssi.2013.05.004
- Tallentire, S., Child, F., Fall, I., Vella-Zarb, L., Evans, I., Tucker, M., …Evans, J. (2013). Systematic and Controllable Negative, Zero, and Positive Thermal Expansion in Cubic Zr1–xSnxMo2O8. Journal of the American Chemical Society, 135(34), 12849-12856. https://doi.org/10.1021/ja4060564
- Ford, S. J., McIntyre, G. J., Johnson, M. R., & Evans, I. R. (2013). Structure and dynamics studies of the short strong hydrogen bond in the 3,5-dinitrobenzoic acid-nicotinic acid molecular complex. CrystEngComm, 15(37), 7576-7582. https://doi.org/10.1039/c3ce40874d
- Kuang, X., Payne, J. L., Farrell, J. D., Johnson, M. R., & Evans, I. R. (2012). Polymorphism and Oxide Ion Migration Pathways in Fluorite-Type Bismuth Vanadate, Bi46V8O89. Chemistry of Materials, 24(11), 2162-2167. https://doi.org/10.1021/cm3008107
- Ling, C., Miiller, W., Johnson, M., Richard, D., Rols, S., Madge, J., & Evans, I. (2012). Local Structure, Dynamics, and the Mechanisms of Oxide Ionic Conduction in Bi26Mo10O69. Chemistry of Materials, 24(23), 4607-4614. https://doi.org/10.1021/cm303202r
- Johnson, R., Tang, C., Evans, I., Bland, S., Free, D., Beale, T., …Boothroyd, A. (2012). X-ray diffraction study of the temperature-induced structural phase transitions in SmVO3. Physical review B, 85(22), Article 224102. https://doi.org/10.1103/physrevb.85.224102
- Kuang, X., Payne, J., Johnson, M., & Evans, I. (2012). Remarkably High Oxide Ion Conductivity at Low Temperature in an Ordered Fluorite-Type Superstructure. Angewandte Chemie International Edition, 51(3), 690-694. https://doi.org/10.1002/anie.201106111
- Coelho, A., Evans, J. S., Evans, I. R., Kern, A., & Parsons, S. (2011). The TOPAS symbolic computation system. Powder Diffraction, 26(S1), S22-S25. https://doi.org/10.1154/1.3661087
- Krstic, M., Sovilj, S. P., Grguric-Sipka, S., Evans, I. R., Borozan, S., & Santibanez, J. F. (2011). Synthesis, structural and spectroscopic characterization, in vitro cytotoxicity and in vivo activity as free radical scavengers of chlorido(p-cymene) complexes of ruthenium(II) containing N-alkylphenothiazines. European Journal of Medicinal Chemistry, 46(9), 4168-4177. https://doi.org/10.1016/j.ejmech.2011.06.019
- Damjanovic, L., Holclajtner-Antunovic, I., Mioc, U. B., Bikic, V., Milovanovic, D., & Evans, I. R. (2011). Archaeometric study of medieval pottery excavated at Stari (Old) Ras, Serbia. Journal of Archaeological Science, 38(4), 818-828. https://doi.org/10.1016/j.jas.2010.11.004
- Ford, S., Delamore, O., Evans, J., McIntyre, G., Johnson, M., & Evans, I. (2011). Giant Deuteron Migration During the Isosymmetric Phase Transition in Deuterated 3,5-Pyridinedicarboxylic Acid. Chemistry - A European Journal, 17(52), 14942-14951. https://doi.org/10.1002/chem.201101104
- Krstic, M., Sovilj, S. P., Grguric-Sipka, S., Evans, I. R., Borozan, S., Santibanez, J. F., & Kocic, J. (2010). New ruthenium(II) complexes with N-alkylphenothiazines: Synthesis, structure, in vivo activity as free radical scavengers and in vitro cytotoxicity. European Journal of Medicinal Chemistry, 45(9), 3669-3676. https://doi.org/10.1016/j.ejmech.2010.05.013
- Kuang, X., & Evans, I. (2010). Four New Dysprosium and Neodymium Octamolybdate Hydrates: Assembly of RE2(Mo8O27) Sheets and Topotactic Transformations. Inorganic Chemistry, 49(13), 6005-6012. https://doi.org/10.1021/ic100646e
- Das, J., Freitas, F., Evans, I., Nogueira, A., & Khushalani, D. (2010). A facile nonaqueous route for fabricating titania nanorods and their viability in quasi-solid-state dye-sensitized solar cells. Journal of materials chemistry, 20(21), 4425-4431. https://doi.org/10.1039/b921373b
- Kuang, X., Li, Y., Ling, C., Withers, R., & Evans, I. (2010). Oxide Ion Conductivity, Phase Transitions, and Phase Separation in Fluorite-Based Bi38-xMo7xO78+1.5x. Chemistry of Materials, 22(15), 4484-4494. https://doi.org/10.1021/cm101324n
- Lister, S. E., Evans, I. R., & Evans, J. S. (2009). Complex Superstructures of Mo2P4O15. Inorganic Chemistry, 48(19), 9271-9281. https://doi.org/10.1021/ic901090p
- Bezjak, J., Recnik, A., Jancar, B., Boullay, P., Evans, I. R., & Suvorov, D. (2009). High-Temperature Transmission Electron Microscopy and X-Ray Powder Diffraction Studies of Polymorphic Phase Transitions in Ba4Nb2O9. Journal of the American Ceramic Society, 92(8), 1806-1812. https://doi.org/10.1111/j.1551-2916.2009.03111.x
- Das, J., Evans, I. R., & Khushalani, D. (2009). Zinc Glycolate: A Precursor to ZnO. Inorganic Chemistry, 48(8), 3508-3510. https://doi.org/10.1021/ic900067w
- Li, Y., Hutchinson, T. P., Kuang, X., Slater, P. R., Johnson, M. R., & Evans, I. R. (2009). Ionic Conductivity, Structure and Oxide Ion Migration Pathway in Fluorite-Based Bi8La10O27. Chemistry of Materials, 21(19), 4661-4668. https://doi.org/10.1021/cm901770n
- Barker, R. S., & Evans, I. R. (2008). Structural characterization of RE(10)W(22)O(81) rare-earth tungstates (RE = Ce, Nd). Acta crystallographica. Section B, Structural science, 64(6), 708-712. https://doi.org/10.1107/s0108768108033430
- Evans, I. R., Howard, J. A., & Evans, J. S. (2008). Structural ferroelectric phase transition and polymorphism in 2-aminopyridine dihydrogen phosphate. Crystal Growth and Design, 8(5), 1635-1639. https://doi.org/10.1021/cg701076s
- Hutchinson, T. P., & Evans, I. R. (2008). Comment on new oxide ion conductors La3MMo2O12 (M = In, Ga, Al). Solid State Ionics, 178(31-32), 1660-1662. https://doi.org/10.1016/j.ssi.2007.10.021
- Crossland, C. J., Evans, I. R., & Evans, J. S. (2008). Structural chemistry of (PPh4)2M(WS4)2 materials. Dalton Transactions, 1597-1601. https://doi.org/10.1039/b716407f
- Evans, I. R., Howard, J. A., Evans, J. S., Postlethwaite, S. R., & Johnson, M. R. (2008). Polymorphism and hydrogen bonding in cinchomeronic acid: a variable temperature experimental and computational study. CrystEngComm, 10(10), 1404-1409. https://doi.org/10.1039/b807015f
- Othman, A., Evans, J. S., Evans, I. R., Harris, R. K., & Hodgkinson, P. (2007). Structural study of polymorphs and solvates of finasteride. Journal of Pharmaceutical Sciences, 96(5), 1380-1397. https://doi.org/10.1002/jps.20940
- Jacimovic, Z. K., Radovic, A., Leovac, V. M., Tomid, Z. D., & Evans, I. R. (2007). Crystal structure of bis(mu(2)-thiocyanato)tetrakis(3,5-dimethyl-t(thiocarbamoy)pyrazole)dini ckel(II) dichloride ethanol disolvate, [Ni(NCS)(2)(C6H6N3S)(4)] [Cl](2)center dot 2C(2)H(5)OH. Zeitschrift für Kristallographie - New Crystal Structures, 222(4), 430-432. https://doi.org/10.1524/ncrs.2007.0182
- Barker, R., & Evans, I. (2006). An investigation of the Nd₂O₃-MoO₃ phase system: thermal decomposition of Nd₂Mo₄O₁₅ and formation of Nd₆Mo₁₀O₃₉. Journal of Solid State Chemistry, 179(6), 1918-1923. https://doi.org/10.1016/j.jssc.2006.03.023
- Russell, J., Parker, A., Radosavljevie-Evans, I., Howard, J., & Steed, J. (2006). Simultaneous anion and cation binding by a simple polymer-bound ureidopyridyl ligand. Chemical Communications, 2006(3), 269-271. https://doi.org/10.1039/b513820e
- Szecseny, K., Leovac, V., & Evans, I. (2006). Synthesis and characterisation of a novel polymeric Cd complex, catena-(mu-thio)[bis(N-phenylthiourea]bis(dimethylsulphoxide)dichloroc admium(II). Journal of Coordination Chemistry, 59(5), 523-530. https://doi.org/10.1080/00958970500171240
- Russell, J., Parker, A., Radosavljevic-Evans, I., Howard, J., & Steed, J. (2006). Anion-binding mode in a sulfanylphenyl urea complex : solid state symmetry breaking and solution chelation. CrystEngComm, 8(2), 119-122. https://doi.org/10.1039/b516962c
- Evans, I., Howard, J., & Evans, J. (2005). The crystal structure of alpha-La2Mo2O9 and the structural origin of the oxide ion migration pathway. Chemistry of Materials, 17(16), 4074-4077. https://doi.org/10.1021/cm050049%2B
- Evans, I., Tao, S., & Irvine, J. (2005). Phase transition, thermal expansion and electrical properties of BiCu2VO6. Journal of Solid State Chemistry, 178, 2927-2933
- Evans, I., Szecsenyi, K., & Leovac, V. (2005). Di-mu-chloro-bis\chloro[3,5-dimethyl-1-(thiocarbamoyl)pyrazole-kappa N-2(2),S]cadmium(II)\. Acta crystallographica. Section E, 61(4), M641-M643. https://doi.org/10.1107/s1600536805006173
- Evans, I., Howard, J., & Sleight, A. (2005). Synthesis and crystal structure of BiCd2AsO6. Solid State Sciences, 7(3), 299-302
- Evans, I., Szecsenyi, K., & Leovac, V. (2005). 1-(hydroxymethyl)-3,5-dimethylpyrazole. Acta crystallographica. Section E, 61(3), O625-O626. https://doi.org/10.1107/s1600536805003971
- Applegarth, L., Clark, N., Richardson, A., Parker, A., Radosavljevic-Evans, I., Goeta, A., …Steed, J. (2005). Modular nanometer-scale structuring of gel fibres by sequential self-organization. Chemical Communications, 5423-5425
- Kovacs, A., Nemcsok, D., Pokol, G., Szecsenyi, K., Leovac, V., Jacimovic, Z., …Giester, G. (2005). Structural, spectroscopic and computational studies of the HgL2Cl2 complex (L=3,5-dimethyl-1-thiocarboxamide pyrazole) and the crystal structure of L. New Journal of Chemistry, 29(6), 833-840
- Turner, D., Smith, B., Spencer, E., Goeta, A., Evans, I., Tocher, D., …Steed, J. (2005). Anion binding by Ag(I) complexes of urea-substituted pyridyl ligands. New Journal of Chemistry, 29(1), 90-98
- Evans, I., Howard, J., Howard, L., Evans, J., Jacimovic, Z., Jevtovic, V., & Leovac, V. (2004). Transition metal complexes with pyrazole based ligands, part 18: new binuclear Cu(I), Cu(II) and Co(II) complexes with 3,5-dimethyl-l-thiocarboxamide pyrazole: synthesis, structural and magnetic studies. Inorganica Chimica Acta, 357(15), 4528-4536
- Rubin-Preminger, J., Bernstein, J., Harris, R., Evans, I., & Ghi, P. (2004). [R,S]-Ethambutol dihydrochloride: Variable-temperature studies of a dimorphic system with very similar packing. Journal of Pharmaceutical Sciences, 93(11), 2810-2819. https://doi.org/10.1002/jps.20136
- Jacimovic, Z., Leovac, V., Szecsenyi, K., Howard, J., & Evans, I. (2004). Transition metal complexes with pyrazole-based ligands. XIX. Diaquabis(3,5-dimethyl-1H-pyrazole-1-carboxamidine-kappa N-2,N `)metal(II) dinitrate, with metal = Co and Ni. Acta crystallographica. Section C, Crystal structure communications, 60(10), M467-M470. https://doi.org/10.1107/s0108270104017433
- Evans, I., Howard, J., Savikin-Fodulovic, K., & Menkovic, N. (2004). Isogentisin (1,3-dihydroxy-7-methoxyxanthone). Acta crystallographica. Section B, Structural science, 60, O1557-O1559
- Rubin-Preminger, J., Bernstein, J., Harris, R., Evans, I., & Ghi, P. (2004). Variable temperature studies of a polymorphic system comprising two pairs of enantiotropically related forms: S,S -ethambutol dihydrochloride. Crystal Growth and Design, 4(3), 431-439. https://doi.org/10.1021/cg0341959
- Evans, I., Howard, J., Szecsenyi, K., Leovac, V., & Jacimovic, Z. (2004). Synthesis, characterization and crystal structure of a novel Ni(II) complex, Ni(L-H)(2) (L=3,5-dimethyl-1-thiocarboxamidopyrazole). Journal of Coordination Chemistry, 57(6), 469-476
- Money, V., Evans, I., Elhaik, J., Halcrow, M., & Howard, J. (2004). An X-ray powder diffraction study of the spin-crossover transition and structure of bis (2,6-dipyrazol-1-ylpyrazine)iron(II) perchlorate. Acta crystallographica. Section B, Structural science, 60, 41-45
- Evans, J., & Evans, I. (2004). Beyond classical applications of powder diffraction. Chemical Society Reviews, 33(8), 539-547
- Turner, D., Smith, B., Goeta, A., Evans, I., Tocher, D., Howard, J., & Steed, J. (2004). The R(1)(2)(6) hydrogen-bonded synthon in neutral urea and metal-bound halide systems. CrystEngComm, 6, 633-641. https://doi.org/10.1039/b415627g
- Money, V., Elhaik, J., Evans, I., Halcrow, M., & Howard, J. (2004). A study of the thermal and light induced spin transition in FeL2 (BF4)(2) and FeL2 (ClO4)(2) L=2,6-di(3-methylpyrazol-1-yl)pyrazine. Dalton Transactions, 43(1), 65-69. https://doi.org/10.1039/b311262b
- Lister, S., Evans, I., Howard, J., Coelho, A., & Evans, J. (2004). Mo2P4O15 - the most complex oxide structure solved by single crystal methods?. Chemical Communications, 2540-2541
- Evans, I., Howard, J., & Evans, J. (2003). alpha-Bi2Sn2O7 - a 176 atom crystal structure from powder diffraction data. Journal of materials chemistry, 13(9), 2098-2103. https://doi.org/10.1039/b305211g
- Evans, I., Howard, J., Sreckovic, T., & Ristic, M. (2003). Variable temperature in situ X-ray diffraction study of mechanically activated synthesis of calcium titanate, CaTiO3. Materials Research Bulletin, 38(7), 1203-1213
- Money, V., Evans, I., Halcrow, M., Goeta, A., & Howard, J. (2003). Light induced excited high spin-state trapping in [FeL2](BF4)(2) (L=2,6-di(pyrazol-1-yl)pyridine). Chemical Communications, 158-159. https://doi.org/10.1039/b210146g
- Elhaik, J., Money, V., Barrett, S., Kilner, C., Evans, I., & Halcrow, M. (2003). The spin-states and spin-crossover behaviour of iron(II) complexes of 2,6-dipyrazol-1-ylpyrazine derivatives. Dalton Transactions, 2053-2060. https://doi.org/10.1039/b210368k
- Evans, I., Tao, S., Irvine, J., & Howard, J. (2002). Synthesis, crystal structure, and oxide ion conductivity in Bi4.6Ca1.1VO10.5. Chemistry of Materials, 14(9), 3700-3704
- Evans, I., & Howard, J. (2002). Lithium potassium tungstate monohydrate, LiKWO4 center dot H2O. Acta crystallographica. Section B, Structural science, 58, i26-I28
- Evans, I., Evans, J., & Howard, J. (2002). Variable temperature structural study of bismuth lead vanadate, BiPb₂VO₆. Journal of materials chemistry, 12(9), 2648-2652. https://doi.org/10.1039/b202648a
- Kumar, V., Addlagatta, A., Nangia, A., Robinson, W., Broder, C., Mondal, R., …Allen, F. (2002). 4,4-diphenyl-2,5-cyclohexadienone: Four polymorphs and nineteen crystallographically independent molecular conformations. Angewandte Chemie International Edition, 41(20), 3848-3851
- Evans, I., Howard, J., Withers, R., & Evans, J. (2001). Ab initio structure determination of BiPb2VO6 from powder diffraction data. Chemical Communications, 1984-1985
- Radosavljevic, I., Howard, J., & Sleight, A. (2000). Synthesis and structure of two new bismuth cadmium vanadates, BiCdVO5 and BiCd2VO6, and structures of BiCa2AsO6 and BiMg2PO6. International journal of inorganic materials, 2(6), 543-550. https://doi.org/10.1016/s1466-6049%2800%2900080-5
- Radosavljevic, I., & Sleight, A. (2000). Variable temperature X-ray diffraction study of bismuth magnesium vanadate, BiMg2VO6. Journal of Solid State Chemistry, 149(1), 143-148. https://doi.org/10.1006/jssc.1999.8512
- Radosavljevic, I., Howard, J., Sleight, A., & Evans, J. (2000). Synthesis and structure of Bi3Ca9V11O41. Journal of materials chemistry, 10(9), 2091-2095. https://doi.org/10.1039/b002857f
- Radosavljevic, I., Evans, J., & Sleight, A. (1999). Synthesis and structure of bismuth copper arsenate, BiCu2AsO6. Journal of Alloys and Compounds, 284(1-2), 99-103. https://doi.org/10.1016/s0925-8388%2898%2900919-0
- Radosavljevic, I., Evans, J., & Sleight, A. (1998). Synthesis and structure of bismuth copper vanadate, BiCu2VO6. Journal of Solid State Chemistry, 141(1), 149-154. https://doi.org/10.1006/jssc.1998.7931
- Radosavljevic, I., Evans, J., & Sleight, A. (1998). Synthesis and structure of pyrochlore-type bismuth titanate. Journal of Solid State Chemistry, 136(1), 63-66. https://doi.org/10.1006/jssc.1997.7655
- Radosavljevic, I., Evans, J., & Sleight, A. (1998). Synthesis and structure of BiCa2VO6. Journal of Solid State Chemistry, 137(1), 143-147. https://doi.org/10.1006/jssc.1997.7741
Report
- Taylor, S., Gasper, G., Uchacz, T., Smithson, H. E., McLeish, T. C., Kollandsrud, K., …Evans, I. (2022). Cultural Heritage 360 A Report for the AHRC Programme: Where Next? Scoping Future Arts and Humanities Led Research. [No known commissioning body]
- Taylor, S., Gasper, G., Uchacz, T., Smithson, H., McLeish, T., Kollandsrud, K., …Evans, I. (2022). Cultural Heritage 360: A Report for the AHRC Programme: Where Next? Scoping Future Arts and Humanities Led Research. Arts and Humanities Research Council

