Staff profile

Biography
Karl graduated with a First Class Chemistry degree and PhD from Leicester University, held an individual Marie Curie Research Fellowship in Strasbourg, France then a Royal Society University Research Fellowship in Chemistry at the University of Oxford. He joined Durham Chemistry in 2004 and was promoted to a Reader in 2010 and Chair in 2012. Karl has held a number of leadership roles including Director of Research in the Chemistry Department and has been Head of Department since August 2017. He represents the Faculty of Science on Senate Agenda Setting Committee.
Karl’s research is focussed on nanomaterials in particular the chemistry of graphene and carbon nanotubes. His work has been recognised with numerous awards, including the international Royal Society of Chemistry Entrepreneur of the Year Award 2011 for his work on graphene, the Times Higher Education Research and Innovation Award 2012 and more recently the Royal Society of Chemistry 2017 Materials for Industry – Derek Birchall Award . He has served as Chair of the Chemical Nanosciences and Nanotechnology subject group of the Royal Society of Chemistry (2016 - 2021) and is an expert member on committee AMT/009 in the BSI, the UK national standards body. Karl recently completed a 5-year term (ended 2019) as a Member of the Digital Programme (formerly Innovation, Research and Development) Expert Group, operated by the UK Government Department for Business, Energy and Industrial Strategy. This involved overseeing progress and offering strategic direction for a number of research programmes across the National Measurement System (NMS) remit (of which the National Physical Laboratory is part).
Karl is the editor of the Elsevier journal FlatChem: Chemistry of 2D Materials and sits on the editorial board of the scientific journals Crystals, ISRN Nanotechnology and the Journal of Carbon Research. Karl established the successful Durham University spinout company Applied Graphene Materials in 2010, which is now listed on the FTSE AIM index, and he is currently the Chief Scientific Officer.
Research Interests
The group’s research interests are in the field of nanomaterials and nanotechnology. Nanotechnology is the science of creating structures or materials on a nanometre (one millionth of a millimetre) scale. Interestingly, the fundamental physical and chemical properties of materials are altered as they are decreased to the nanometre scale. Therefore, nanostructured materials offer great potential in the development of new electronic devices, bio-sensors and high strength composites. Our work in this area involves, amongst others, the synthesis and chemistry of graphene and carbon nanotubes. We use chemistry to improve the compatibility and dispersion in a range of matrices to facilitate the use of nanocarbons in a range of applications including advanced composites and coatings.
Synthetic procedures within the group often involve sensitive materials which are handled using inert atmosphere glove-box or Schlenk line techniques. As well as making use of the more routine analytical techniques to characterise the materials, such as NMR spectroscopy and mass spectrometry, the group makes extensive use of scanning probe microscopy (SPM), transmission electron microscopy (TEM), scanning electron microscopy (SEM), Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). An outline of some of our interests are given below.
Graphene

Graphene is single layer of carbon atoms arranged in a continuous honeycomb network and is the latest addition to the nanocarbon family. This 2D nanostructure, best visualised as single layer of graphite, shares the exciting properties of other carbon nanomaterials. Like carbon nanotubes, which can be considered to be a rolled up sheet of graphene, the material has exceptional electrical, thermal and mechanical properties. As a result various applications in materials science including polymer nanocomposites, energy storage materials, transparent thin film electrodes and nanoelectronic components have been envisaged. It has even been suggested that graphene could outperform carbon nanotubes in some of these applications.
One of the problems in graphene research is the availability of the material and the difficulties involved with its synthesis. These issues need to be solved if the applications listed above are to be made viable. Our interests lie in the synthesis of graphene using a variety of methodologies that are scalable and selective for the formation of graphene or few-layer graphene. We are particularly interested in producing graphene foams for energy storage applications where pore size can be controlled.
We are also investigating methods of chemically functionalising graphene to improve and control dispersion in aqueous and non-aqueous solvents. We are particularly interested in using graphene and chemically modified graphene in applications such as coatings and composites.
We formed a University spinout company Durham Graphene Science, now named Applied Graphene Materials plc (http://www.appliedgraphenematerials.com) to commercialise some aspects of this work. Applied Graphene Materials plc is now listed on the FTSE AIM market.
Chemistry of Carbon Nanotubes
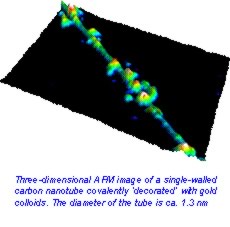
Single-walled carbon nanotubes (SWNTs) have attracted interest and excitement across a broad spectrum of sciences from engineering, materials, chemistry, biology to medicine. Single-walled carbon nanotubes can simply be thought of as a rolled up single sheet of graphite joined at the edges. They are immensely strong with a strength similar to that of steel and can be metallic or semi-conducting depending on their structure. Such impressive mechanical and electronic properties have opened the way for the development of new technologies. However, many possible applications of nanotubes, from use as components in electronics to chemical and biological sensors, can only be realized through chemical control.
We are currently investigating methods of chemically functionalising the carbon nanotubes to:
- improve dispersion in aqueous and non-aqueous solvents.
- control their electronic properties for nanoelectronics.
- enhance their interaction with a range of polymer matrices to form new generation nanocomposites.
- improve and tailor the bio- compatibility of the nanotube surface to selectively adsorb biological materials for nanoscale biosensors.
- translocate into cells for imaging and drug delivery.
Key References
- Controlled Structure Evolution of Graphene Networks in Polymer Composites. S. C. Boothroyd, D. W. Johnson, M. P. Weir, C. D. Reynolds, J. M. Hart, A. J. Smith, N. Clarke, R. L. Thompson and K. S. Coleman. Chemistry of Materials 2018, 30, 1524.
- Extrinsic Wrinkling and Single Exfoliated Sheets of Graphene Oxide in Polymer Composites. M. P. Weir, D. W. Johnson, S. C. Boothroyd, R. C. Savage, R. L. Thompson, S. R. Parnell, A. J. Parnell, S. M. King, S. E. Rogers, K. S. Coleman and N. Clarke. Chemistry of Materials 2016, 28, 1698.
- A Manufacturing Perspective on Graphene Dispersions. D. W. Johnson, B. P. Dobson and K. S. Coleman. Current Opinion in Colloid & Interface Science 2015, 20, 367.
- Graphene Film Growth on Polycrystalline Metals. R. S. Edwards and K. S. Coleman. Accounts of Chemical Research 2013, 46, 23.
- Graphene synthesis: relationship to applications. R. S. Edwards and K. S. Coleman. Nanoscale 2013, 5, 38.
- Unweaving the rainbow: a review of the relationship between single-walled carbon nanotube molecular structures and their chemical reactivity. S. A. Hodge, M. K. Bayazit, K. S. Coleman and M. S. P. Shaffer. Chemical Society Reviews 2012, 41, 4409.
- Simple and scalable route for the 'bottom-up' synthesis of few-layer graphene platelets and thin films. C. R. Herron, R. S. Edwards, K. S. Coleman, B. Mendis, Journal of Materials Chemistry 2011, 21, 3378.
- Pyridine-functionalized single-Walled carbon nanotubes as gelators for poly(acrylic acid) hydrogels. M. K. Bayazit, L. S. Clarke, N. Clarke, K. S. Coleman, Journal of the American Chemical Society 2010, 132, 15814.
- Fluorescent single-walled carbon nanotubes following the 1,3-dipolar cycloaddition of pyridinium ylides. M. K. Bayazit, K. S. Coleman. Journal of the American Chemical Society 2009, 131, 10670.
- A facile, solvent-free, noncovalent, and nondisruptive route to functionalize single-wall carbon nanotubes using tertiary phosphines. A. Suri, A. K. Chakraborty, K. S. Coleman. Chemistry of Materials 2008, 20, 1705.
Research interests
- Graphene
- Carbon Nanotubes
- Nanomaterials
- Nanotechnology
Publications
Chapter in book
- Coleman, K. S. (2009). Nanotubes. In F. Berry, & E. Hope (Eds.), ANNUAL REPORTS ON THE PROGRESS OF CHEMISTRY, VOL 105, SECTION A: INORGANIC CHEMISTRY (382-396). Royal Society of Chemistry. https://doi.org/10.1039/b818292m
- Coleman, K. S. (2007). Nanotubes. In F. Berry, & E. Hope (Eds.), ANNUAL REPORTS ON THE PROGRESS OF CHEMISTRY, VOL 103, SECTION A: INORGANIC CHEMISTRY (392-406). Royal Society of Chemistry. https://doi.org/10.1039/b705543a
Journal Article
- Goldie, S. J., & Coleman, K. S. (2023). Graphitization by Metal Particles. ACS Omega, 8(3), 3278-3285. https://doi.org/10.1021/acsomega.2c06848
- Goldie, S. J., Degiacomi, M. T., Jiang, S., Clark, S. J., Erastova, V., & Coleman, K. S. (2022). Identification of Graphene Dispersion Agents through Molecular Fingerprints. ACS Nano, 16(10), https://doi.org/10.1021/acsnano.2c04406
- Goldie, S. J., Jiang, S., & Coleman, K. S. (2021). Cobalt nanoparticle catalysed graphitization and the effect of metal precursor decomposition temperature. Materials Advances, 2(10), 3353-3361. https://doi.org/10.1039/d1ma00125f
- Goldie, S. J., Bush, S., Cumming, J. A., & Coleman, K. S. (2020). Statistical Approach to Raman Analysis of Graphene-Related Materials: Implications for Quality Control. ACS Applied Nano Material, 3(11), 11229-11239. https://doi.org/10.1021/acsanm.0c02361
- Boothroyd, S. C., Johnson, D. W., Weir, M. P., Reynolds, C. D., Hart, J. M., Smith, A. J., …Coleman, K. S. (2018). Controlled Structure Evolution of Graphene Networks in Polymer Composites. Chemistry of Materials, 30(5), 1524-1531. https://doi.org/10.1021/acs.chemmater.7b04343
- Bayazit, M. K., Moniz, S. J., & Coleman, K. S. (2017). Gram-scale production of nitrogen doped graphene using a 1,3-dipolar organic precursor and its utilisation as a stable, metal free oxygen evolution reaction catalyst. Chemical Communications, 53(55), 7748-7751. https://doi.org/10.1039/c7cc04044j
- Tynan, M., Johnson, D., Dobson, B., & Coleman, K. (2016). Formation of 3D Graphene Foams on Soft Templated Metal Monoliths. Nanoscale, 8(27), 13303-13310. https://doi.org/10.1039/c6nr02455f
- Weir, M. P., Johnson, D. W., Boothroyd, S. C., Savage, R. C., Thompson, R. L., Parnell, S. R., …Clarke, N. (2016). Extrinsic Wrinkling and Single Exfoliated Sheets of Graphene Oxide in Polymer Composites. Chemistry of Materials, 28(6), 1698-1704. https://doi.org/10.1021/acs.chemmater.5b04502
- Weir, M., Johnson, D., Boothroyd, S., Savage, R., Thompson, R., King, S., …Clarke, N. (2016). Distortion of Chain Conformation and Reduced Entanglement in Polymer–Graphene Oxide Nanocomposites. ACS Macro Letters, 5(4), 430-434. https://doi.org/10.1021/acsmacrolett.6b00100
- Johnson, D., Dobson, B., & Coleman, K. (2015). A Manufacturing Perspective on Graphene Dispersions. Current Opinion in Colloid and Interface Science, 20(5-6), 367-382. https://doi.org/10.1016/j.cocis.2015.11.004
- Kataky, R., Hadden, J., Coleman, K., Ntola, C., Chowdhury, M., Duckworth, A., …Shenton, F. (2015). Graphene oxide nanocapsules within silanized hydrogels suitable for electrochemical pseudocapacitors. Chemical Communications, 51(51), 10345-10348. https://doi.org/10.1039/c5cc00968e
- Bayazit, M., Pålsson, L., & Coleman, K. (2015). Sensing properties of light-emitting single walled carbon nanotubes prepared via click chemistry of ylides bound to the nanotube surface. RSC Advances, 5(46), 36865-36873. https://doi.org/10.1039/c5ra04330a
- Volpati, D., Massey, M., Johnson, D., Kotsialos, A., Qaiser, F., Pearson, C., …Petty, M. (2015). Exploring the alignment of carbon nanotubes dispersed in a liquid crystal matrix using coplanar electrodes. Journal of Applied Physics, 117(12), Article 125303. https://doi.org/10.1063/1.4916080
- Bayazit, M. K., Celebi, N., & Coleman, K. S. (2014). A theoretical and experimental exploration of the mechanism of microwave assisted 1,3-dipolar cycloaddition of pyridinium ylides to single walled carbon nanotubes. Materials Chemistry and Physics: Including Materials Science Communications, 145(1-2), 99-107. https://doi.org/10.1016/j.matchemphys.2014.01.045
- Jombert, A. S., Bayazit, M. K., Herron, C. R., Coleman, K. S., & Zeze, D. A. (2013). Synthesis and Characterization of Molecularly-Bridged Single-Walled Carbon Nanotubes and Electrical Properties of Their Films. Science of Advanced Materials, 5(12), 1967-1973. https://doi.org/10.1166/sam.2013.1664
- Edwards, R. S., & Coleman, K. S. (2013). Graphene Film Growth on Polycrystalline Metals. Accounts of Chemical Research, 46(1), 23-30. https://doi.org/10.1021/ar3001266
- Edwards, R. S., & Coleman, K. S. (2013). Graphene synthesis: relationship to applications. Nanoscale, 5(1), 38-51. https://doi.org/10.1039/c2nr32629a
- Bayazit, M. K., & Coleman, K. S. (2012). Probing the Selectivity of Azomethine Imine Cycloaddition to Single-Walled Carbon Nanotubes by Resonance Raman Spectroscopy. Chemistry - An Asian Journal, 7(12), 2925-2930. https://doi.org/10.1002/asia.201200714
- Suri, A., & Coleman, K. S. (2012). Formylation of Single-Walled Carbon Nanotubes. Journal of Nanoscience and Nanotechnology, 12(3), 2929-2933. https://doi.org/10.1166/jnn.2012.5696
- Hodge, S. A., Bayazit, M. K., Coleman, K. S., & Shaffer, M. S. (2012). Unweaving the rainbow: a review of the relationship between single-walled carbon nanotube molecular structures and their chemical reactivity. Chemical Society Reviews, 41(12), 4409-4429. https://doi.org/10.1039/c2cs15334c
- Suri, A., & Coleman, K. S. (2011). The superiority of air oxidation over liquid-phase oxidative treatment in the purification of carbon nanotubes. Carbon, 49(9), 3031-3038. https://doi.org/10.1016/j.carbon.2011.03.023
- Dastgir, S., Coleman, K. S., & Green, M. L. (2011). Heterogenised N-heterocyclic carbene complexes: synthesis, characterisation and application for hydroformylation and C-C bond formation reactions. Dalton Transactions, 40(3), 661-672. https://doi.org/10.1039/c0dt00760a
- Herron, C., Coleman, K., Edwards, R., & Mendis, B. (2011). Simple and scalable route for the 'bottom-up' synthesis of few-layer graphene platelets and thin films. Journal of materials chemistry, 21(10), 3378-3383. https://doi.org/10.1039/c0jm03437a
- Bayazit, M. K., Suri, A., & Coleman, K. S. (2010). Formylation of single-walled carbon nanotubes. Carbon, 48(12), 3412-3419. https://doi.org/10.1016/j.carbon.2010.05.036
- Dastgir, S., Coleman, K. S., Cowley, A. R., & Green, M. L. (2010). Synthesis, Structure, and Temperature-Dependent Dynamics of Neutral Palladium Allyl Complexes of Annulated Diaminocarbenes and Their Catalytic Application for C-C and C-N Bond Formation Reactions. Organometallics, 29(21), 4858-4870. https://doi.org/10.1021/om1000327
- Bayazit, M., Clarke, L., Coleman, K., & Clarke, N. (2010). Pyridine-Functionalized Single-Walled Carbon Nanotubes as Gelators for Poly(acrylic acid) Hydrogels. Journal of the American Chemical Society, 132(44), 15814-15819. https://doi.org/10.1021/ja1076662
- Mabrook, M., Jombert, A., Machin, S., Pearson, C., Kolb, D., Coleman, K., …Petty, M. (2009). Memory effects in MIS structures based on silicon and polymethylmethacrylate with nanoparticle charge-storage elements. Materials Science and Engineering: B, 159-160, 14-17. https://doi.org/10.1016/j.mseb.2008.09.003
- Chakraborty, A. K., Coleman, K. S., & Dhanak, V. R. (2009). The electronic fine structure of 4-nitrophenyl functionalized single-walled carbon nanotubes. Nanotechnology, 20(15), Article 155704. https://doi.org/10.1088/0957-4484/20/15/155704
- Dastgir, S., Coleman, K. S., Cowley, A. R., & Green, M. L. (2009). Stable crystalline annulated diaminocarbenes: coordination with rhodium(I), iridium(I) and catalytic hydroformylation studies. Dalton Transactions, 7203-7214. https://doi.org/10.1039/b905729c
- Bayazit, M., & Coleman, K. (2009). Fluorescent Single-Walled Carbon Nanotubes Following the 1,3-Dipolar Cycloaddition of Pyridinium Ylides. Journal of the American Chemical Society, 131(30), 10670-10676. https://doi.org/10.1021/ja903712f
- Jombert, A., Coleman, K., Wood, D., Petty, M., & Zeze, D. (2008). Poole-Frenkel conduction in single wall carbon nanotube composite films built up by electrostatic layer-by-layer deposition. Journal of Applied Physics, 104(9), Article 094503. https://doi.org/10.1063/1.3006015
- Chakraborty, A. K., & Coleman, K. S. (2008). Poly(ethylene) Glycol/Single-Walled Carbon Nanotube Composites. Journal of Nanoscience and Nanotechnology, 8(8), 4013-4016. https://doi.org/10.1166/jnn.2008.an43
- Watson, S., Coleman, K., & Chakraborty, A. (2008). A new route to the production and nanoscale patterning of highly smooth, ultrathin zirconium oxide films. ACS Nano, 2(4), 643-650. https://doi.org/10.1021/nn700138q
- Suri, A., Chakraborty, A., & Coleman, K. (2008). A facile, solvent-free, noncovalent, and nondisruptive route to functionalize single-wall carbon nanotubes using tertiary phosphines. Chemistry of Materials, 20(5), 1705-1709. https://doi.org/10.1021/cm071573e
- Mahapatra, R., Chakraborty, A., Horsfall, A., Wright, N., Beamson, G., & Coleman, K. (2008). Energy-band alignment of HfO2/SiO2/SiC gate dielectric stack. Applied Physics Letters, 92(4), Article 042904. https://doi.org/10.1063/1.2839314
- Mahapatra, R., Chakraborty, A., Horsfall, A., Chattopadhyay, S., Wright, N., & Coleman, K. (2007). Effects of interface engineering for HfO2 gate dielectric stack on 4H-SiC. Journal of Applied Physics, 102(2), Article 024105. https://doi.org/10.1063/1.2756521
- Das, K., Chakraborty, A., NandaGoswami, M., Shingha, R., Dhar, A., Coleman, K., & Ray, S. (2007). Temperature dependent shape transformation of Ge nanostructures by the vapor-liquid-solid method. Journal of Applied Physics, 101(7), Article 074307. https://doi.org/10.1063/1.2718282
- Coleman, K., Chakraborty, A., Bailey, S., Sloan, J., & Alexander, M. (2007). Iodination of single-walled carbon nanotubes. Chemistry of Materials, 19(5), 1076-1081. https://doi.org/10.1021/cm062730x
- Mahapatra, R., Chakraborty, A., Poolamai, N., Horsfall, A., Chattopadhyay, S., Wright, N., …Burrows, C. (2007). Leakage current and charge trapping behavior in TiO[sub 2]∕SiO[sub 2] high-κ gate dielectric stack on 4H-SiC substrate. Journal of vacuum science & technology. B, Microelectronics and nanometer structures, 25(1), 217-223. https://doi.org/10.1116/1.2433976
- Suri, A., & Coleman, K. S. (2007). INOR 1008-New, versatile route for chemical modification of carbon nanotubes while preserving intrinsic electronic structure
- Watson, S. M., & Coleman, K. S. (2007). COLL 92-Production and nanopatterning of ultra-thin metal oxide films, by scanning probe lithography
- Davis, J., Bagshaw, C., Busuttil, K., Hanyu, Y., & Coleman, K. (2006). Spatially controlled Suzuki and Heck catalytic molecular coupling. Journal of the American Chemical Society, 128(43), 14135-14141. https://doi.org/10.1021/ja064840a
- Palumbo, M., Lee, K., Ahn, B., Suri, A., Coleman, K., Zeze, D., …Petty, M. (2006). Electrical investigations of layer-by-layer films of carbon nanotubes. Journal of Physics D: Applied Physics, 39(14), 3077-3085. https://doi.org/10.1088/0022-3727/39/14/030
- Flahaut, E., Sloan, J., Friedrichs, S., Kirkland, A., Coleman, K., Williams, V., …Green, M. (2006). Crystallization of 2H and 4H PbI2in Carbon Nanotubes of Varying Diameters and Morphologies. Chemistry of Materials, 18(8), 2059-2069. https://doi.org/10.1021/cm0526056
- Mahapatra, R., Poolamai, N., Chattopadhyay, S., Wright, N., Chakraborty, A., Coleman, K., …Burrows, C. (2006). Characterization of thermally oxidized Ti/SiO2 gate dielectric stacks on 4H-SiC substrate. Applied Physics Letters, 88(7), https://doi.org/10.1063/1.2173713
- Dastgir, S., Coleman, K., Cowley, A., & Green, M. (2006). A stable crystalline imino-N-heterocyclic carbene ligand and its corresponding palladium(II) and rhodium(I) complexes. Organometallics, 25(1), 300-306. https://doi.org/10.1021/om050775m
- Davis, J., Coleman, K., Busuttil, K., & Bagshaw, C. (2005). Spatially resolved Suzuki coupling reaction initiated and controlled using a catalytic AFM probe. Journal of the American Chemical Society, 127(38), 13082-13083. https://doi.org/10.1021/ja043235%2B
- Costa, P., Coleman, K., & Green, M. (2005). Influence of catalyst metal particles on the hydrogen sorption of single-walled carbon nanotube materials. Nanotechnology, 16(4), 512-517
- Pascu, S., Coleman, K., Cowley, A., Green, M., & Rees, N. (2005). New cationic palladium (II) and rhodium (I) complexes of [Ph2PCH2C(Ph)=N(2,6-Me2C6H3)]. Journal of Organometallic Chemistry, 690(6), 1645-1658
- Coleman, K., Dastgir, S., Barnett, G., Alvite, M., Cowley, A., & Green, M. (2005). A nonenolizable imino-N-heterocyclic carbene ligand and corresponding silver(I) metal complex. Journal of Organometallic Chemistry, 690, 5591-5596
- Coleman, K., Turberville, S., Pascu, S., & Green, M. (2005). Synthesis of a new bidentate ferrocenyl N-heterocyclic carbene ligand precursor and the palladium (II) complex trans-[PdCl2(C over cap fc over cap C)], where (C over cap fc over cap C)=1,1 `-di-tert-butyl-3,3 ` (1,1 `-dimethyleneferrocenyl)-diimidazol-2-ylidene. Journal of Organometallic Chemistry, 690(3), 653-658. https://doi.org/10.1016/j.jorganchem.2004.10.019
- Pascu, S., Coleman, K., Cowley, A., Green, M., & Rees, N. (2005). New group 10 complexes of the bulky iminophosphine ligands[Ph2PCH2C(Ph)=N(2,6-R2C6H3)], where R = Me, Pr-i. New Journal of Chemistry, 29(2), 385-397
- Coleman, K., Turberville, S., Pascu, S., & Green, M. (2004). Synthesis of a new zwitterionic cyclopentadienyl-imidazolium compound and isolation of the 3,3'-(trans-3,5-cyclopentenyl)-di(1-tert-butylimidazolium)bromide intermediate. Tetrahedron Letters, 45(47), 8695-8698. https://doi.org/10.1016/j.tetlet.2004.09.137
- Coleman, K., Turberville, S., Pascu, S., & Green, M. (2004). Silicon containing ferrocenyl phosphane ligands. Journal of Organometallic Chemistry, 689(4), 770-774
- Coleman, K., Bailey, S., Fogden, S., & Green, M. (2003). Functionalization of single-walled carbon nanotubes via the Bingel reaction. Journal of the American Chemical Society, 125(29), 8722-8723. https://doi.org/10.1021/ja0355675
- Coleman, K., Chamberlayne, H., Turberville, S., Green, M., & Cowley, A. (2003). Silver(I) complex of a new imino-N-heterocyclic carbene and ligand transfer to palladium(II) and rhodium(I). Dalton Transactions, 2917-2922
- Brown, G., Bailey, S., Novotny, M., Carter, R., Flahaut, E., Coleman, K., …Sloan, J. (2003). High yield incorporation and washing properties of halides incorporated into single walled carbon nanotubes. Applied Physics A: Materials Science and Processing, 76(4), 457-462
- Davis, J., Coleman, K., Azamian, B., Bagshaw, C., & Green, M. (2003). Chemical and biochemical sensing with modified single walled carbon nanotubes. Chemistry - A European Journal, 9, 3732-3739
- Xiao, T., Wang, H., Da, J., Coleman, K., & Green, M. (2002). Study of the preparation and catalytic performance of molybdenum carbide catalysts prepared with C2H2/H-2 carburizing mixture. Journal of Catalysis, 211(1), 183-191
- Azamian, B., Davis, J., Coleman, K., Bagshaw, C., & Green, M. (2002). Bioelectrochemical single-walled carbon nanotubes. Journal of the American Chemical Society, 124(43), 12664-12665
- Coleman, K., Sloan, J., Hanson, N., Brown, G., Clancy, G., Terrones, M., …Green, M. (2002). The formation of ReS2 inorganic fullerene-like structures containing Re4 parallelogram units and metal-metal bonds. Journal of the American Chemical Society, 124(39), 11580-11581. https://doi.org/10.1021/ja0261630
- Xu, C., Flahaut, E., Bailey, S., Brown, G., Sloan, J., Coleman, K., …Green, M. (2002). Purification of single-walled carbon nanotubes grown by a chemical vapour deposition (CVD) method. Chemical Research in Chinese Universities, 18(2), 130-132
- Ji, S., Xiao, T., Li, S., Xu, C., Hou, R., Coleman, K., & Green, M. (2002). The relationship between the structure and the performance of Na-W-Mn/SiO2 catalysts for the oxidative coupling of methane. Applied Catalysis A: General, 225(1-2), 271-284
- Azamian, B., Coleman, K., Davis, J., Hanson, N., & Green, M. (2002). Directly observed covalent coupling of quantum dots to single-wall carbon nanotubes. Chemical Communications, 366-367
- Coleman, K., Fawcett, J., Holloway, J., Hope, E., & Nassar, R. (2001). A new synthetic route to [MF(mu-F)(CO)(3)](4) (M = Ru, Os) and their reactivity with P(C6H4-4-X)(3) (X = OCH3, OH) and P(C6H4-2-OH)(3) -Crystal structure of [OS(CO)(3){kappa(3)-(2-OC6H4)(2)P(C6H4-2-OH)}]center dot C3H6O. Journal of Fluorine Chemistry, 112(2), 185-189. https://doi.org/10.1016/s0022-1139%2801%2900509-7
- Xiao, T., Ji, S., Wang, H., Coleman, K., & Green, M. (2001). Methane combustion over supported cobalt catalysts. Journal of Molecular Catalysis A: Chemical, 175(1-2), 111-123
- Xiao, T., York, A., Coleman, K., Claridge, J., Sloan, J., Charnock, J., & Green, M. (2001). Effect of carburising agent on the structure of molybdenum carbides. Journal of materials chemistry, 11(12), 3094-3098
- Brown, G., Bailey, S., Sloan, J., Xu, C., Friedrichs, S., Flahaut, E., …Green, M. (2001). Electron beam induced in situ clusterisation of 1D ZrCl4 chains within single-walled carbon nanotubes. Chemical Communications, 845-846
- Coleman, K., Green, M., Pascu, S., Rees, N., Cowley, A., & Rees, L. (2001). Palladium(II) complexes with the bidentate iminophosphine ligand[Ph2PCH2C(Ph)=N(2,6-Me2C6H3)]. Journal of the Chemical Society. Dalton transactions (2001. Online), 3384-3395. https://doi.org/10.1039/b102476k
- Ji, S., Xiao, T., Wang, H., Flahaut, E., Coleman, K., & Green, M. (2001). Catalytic combustion of methane over cobalt-magnesium oxide solid solution catalysts. Catalysis Letters, 75(1-2), 65-71
- Sloan, J., Novotny, M., Bailey, S., Brown, G., Xu, C., Williams, V., …Hutchison, J. (2000). Two layer 4 : 4 co-ordinated KI crystals grown within single walled carbon nanotubes. Chemical Physics Letters, 329(1-2), 61-65
- Coleman, K., Bedel, L., & Osborn, J. (2000). Catalytic oxidation of alcohols to aldehydes or ketones using osmium-oxo complexes with sulfoxides or N-methylmorpholine-N-oxide asthe co-oxidant: a comparative study. Comptes rendus de l'Académie des sciences. Série IIc, Chimie, 3(10), 765-769. https://doi.org/10.1016/s1387-1609%2800%2901201-9
- Bellemin-Laponnaz, S., Coleman, K., Dierkes, P., Masson, J., & Osborn, J. (2000). Synthesis and coordination of the new chiral tridentate O,N,O ligand2,6-[bis[(1S,2S,5R)-(-)-menthyl]pyridine to molybdenum(VI) andvanadium(V) oxo complexes: Crystal structures of [(2,6-bis{(-)-menthyl}pyridine)MoO2] and [(2,6-bis{(-)-menthyl}pyridine)VO](. European Journal of Inorganic Chemistry, 1645-1649. https://doi.org/10.1002/1099-0682%28200007%292000%3A7%3C1645%3A%3Aaid-ejic1645%3E3.0.co%3B2-h
- Xu, C., Sloan, J., Brown, G., Bailey, S., Williams, V., Friedrichs, S., …Green, M. (2000). 1D lanthanide halide crystals inserted into single-walled carbon nanotubes. Chemical Communications, 2427-2428
- Sloan, J., Dunin-Borkowski, R., Hutchison, J., Coleman, K., Williams, V., Claridge, J., …Green, M. (2000). The size distribution, imaging and obstructing properties of C-60 and higher fullerenes formed within arc-grown single walled carbon nanotubes. Chemical Physics Letters, 316(3-4), 191-198
- Coleman, K., Coppe, M., Thomas, C., & Osborn, J. (1999). Catalytic oxidation of alcohols into aldehydes and ketones by anosmium-copper bifunctional system using molecular oxygen. Tetrahedron Letters, 40(19), 3723-3726
- Clark, H., Coleman, K., Fawcett, J., Holloway, J., Hope, E., Redding, J., & Russell, D. (1999). Synthesis and characterisation of [OC-6-33][OsCl2(CO)(2)L-2](L=phosphine). Crystal structure of [OC-6-33][OsCl2(CO)(2)(PEt3)(2)]. Polyhedron, 18(8-9), 1207-1210
- Bellemin-Laponnaz, S., Coleman, K., & Osborn, J. (1999). Co-ordination of the chiral N,O-ligand 2-[(1S, 2S,5R)(-)-menthol]pyridine to molybdenum(VI) and vanadium(IV) oxocomplexes - Crystal structures of [MoO2{2-(-)-menthol-pyridine}(2)] and [VO{2-(-)menthol-pyridine}(2)]. Polyhedron, 18(19), 2533-2536
- Sloan, J., Wright, D., Woo, H., Bailey, S., Brown, G., York, A., …Green, M. (1999). Capillarity and silver nanowire formation observed in single walled carbon nanotubes. Chemical Communications, 699-700
- Coleman, K., Lorber, C., & Osborn, J. (1998). Selective catalytic oxidation of alcohols by a ruthenium-copper bifunctional system using molecular oxygen. European Journal of Inorganic Chemistry, 1673-1675
- Clark, H., Coleman, K., Fawcett, J., Holloway, J., Hope, E., Langer, J., & Smith, I. (1998). Reactions of iridium and rhodium hydrides with anhydrous HF; crystal structure of [Rh(CO)(PPh3)(3)][BF4].thf. Journal of Fluorine Chemistry, 91(2), 207-211. https://doi.org/10.1016/s0022-1139%2898%2900227-9
- Coleman, K., Holloway, J., Hope, E., & Langer, J. (1997). Reaction of ruthenium(II) and osmium(II) hydrides with anhydrous HF. Journal of the Chemical Society. Dalton transactions (2001. Online), 4555-4559. https://doi.org/10.1039/a705582j
- Coleman, K., Fawcett, J., Holloway, J., Hope, E., & Russell, D. (1997). Air-stable ruthenium(II) and osmium(II) fluoride complexes. Crystal structures of [OC-6-13][MF2(CO)(2)(PR3)(2)] [M = Ru, PR3 = PEtPh2; M =Os, PR3 = PPh3 or P(C6H11)(3)]. Journal of the Chemical Society. Dalton transactions (2001. Online), 3557-3562. https://doi.org/10.1039/a702142i
- Coleman, K., Holloway, J., & Hope, E. (1997). Synthesis and characterisation of ruthenium carbonyl fluorides. Journal of the Chemical Society. Dalton transactions (2001. Online), 1713-1717. https://doi.org/10.1039/a608357i
- Atherton, M., Coleman, K., Fawcett, J., Holloway, J., Hope, E., Karacar, A., …Saunders, G. (1995). Pentafluorophenylphosphine complexes of Rhodium(I): Extended x-rayabsorption fine structure studies of[{Rh[PPh(x)(C6F5)(3-x)](2)(mu-Cl)}(n)] (x=0-2) and[{Rh[(C6F5)(2)PCH2CH2P(C6F5)(2)](mu-Cl)}(2)]. Crystal structures of[RhCl(PPh(3)){(C6F5)(2)PCH2CH2P(C6F5. Journal of the Chemical Society. Dalton transactions, 4029-4037
- Coleman, K., Holloway, J., Hope, E., Russell, D., & Saunders, G. (1995). Tris(2,6-Difluorophenyl)phosphite complexes of platinum group metals -Structure of trans-PtCl2(PEt(3))(P(O-2,6-C6H3F2)(3)). Polyhedron, 14(15-16), 2107-2113
- Brewer, S., Coleman, K., Fawcett, J., Holloway, J., Hope, E., Russell, D., & Watson, P. (1995). Ruthenium and osmium acyl fluoride complexes - Crystal structure of [OC-6-13][RUF2(CO)(2)(PPh(3))].CD2Cl(2). Journal of the Chemical Society. Dalton transactions (2001. Online), 1073-1076. https://doi.org/10.1039/dt9950001073

