Materials for Organic Light-Emitting Devices (OLEDs)
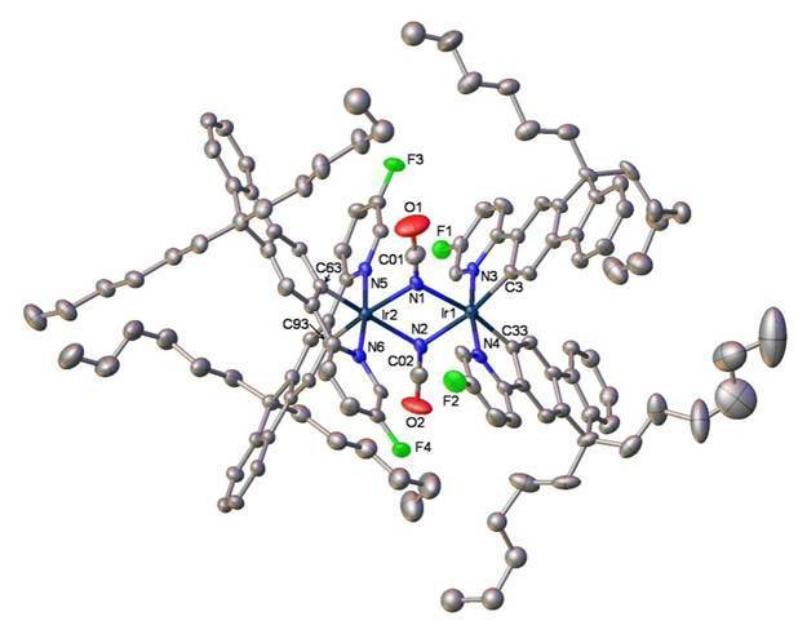
Figure 1. X-Ray molecular structure of a new di-iridium complex for high efficiency green electrophosphorescent devices
The ability to control the optoelectronic properties of conjugated molecular and polymeric systems is a fascinating issue in the design of new materials for light-emitting devices and displays that offer bright colours and a high degree of resolution. Deep blue fluorescent and phosphorescent emitters and white light devices (WOLEDs)1 are of particular current interest. Representative recent work in our laboratory concerns bipolar carbazole-oxadiazole molecules 12 and 2,3 and oligomers and polymers based on hybrid fluorene–dibenzothiophene-S,S-dioxide systems, e.g. 3.4 These materials are used as electroluminescent and electron-transporting layers. New cyclometallated iridium complexes, e.g. 4, are being studied in high-efficiency light-emitting electrochemical cells.5 We have also developed new phosphorescent green and blue emitters based on homoleptic and heteroleptic cyclometallated Ir complexes of heterocyclic ligands,6 e.g. 5. This is interdisciplinary work involving close collaboration with colleagues in the School of Engineering (Professor M. C. Petty) and Department of Physics (Professor A. P. Monkman) in Durham, and with our industrial sponsors who are commercialising some of these materials.
- K. T. Kamtekar, A. P. Monkman, M. R. Bryce, Adv. Mater. 2010, 22, 572.A. L. Fisher, K. E. Linton, K. T. Kamtekar, C. Pearson, M. R. Bryce, M. C. Petty, Chem. Mater. 2011, 23, 1640-1642.
- A. L. Fisher, K. E. Linton, K. T. Kamtekar, C. Pearson, M. R. Bryce, M. C. Petty, Chem. Mater. 2011, 23, 1640-1642.
- Y. Zheng, A. S. Batsanov, V. Jankus, F. B. Dias, M. R. Bryce, A. P. Monkman, J. Org. Chem. 2011, 76, 8300-8310.
- I. I. Perepichka, I. F. Perepichka, M. R. Bryce, L.-O. Palsson, Chem. Commun. 2005, 3397; S. M. King, I. I. Perepichka, I. F. Perepichka, F. B. Dias, M. R. Bryce, A. P. Monkman, Adv. Funct. Mater. 2009, 19, 586; H. Li, A. S. Batsanov, K. C. Moss, H. L. Vaughan, F. B. Dias, K. T. Kamtekar, M. R. Bryce, A. P. Monkman, Chem. Commun. 2010, 46, 4812-4814; K. T. Kamtekar, H. L. Vaughan, B. P. Lyons, A. P. Monkman, S. U. Pandya, M. R. Bryce, Macromolecules 2010, 43, 4481-4488.
- X. Zeng, M. Tavasli, I. F. Perepichka, A. S. Batsanov, M. R. Bryce, C.-J. Chiang, C. Rothe, A. P. Monkman, Chem. Eur. J. 2008, 14, 933; C. Rothe, C.-J. Chiang, V. Jankus, M. R. Bryce, X. Zeng and A. P. Monkman, Adv. Funct. Mater. 2009, 19, 2038-2044.
- Y. Zheng, A. S. Batsanov, M. R. Bryce, Inorg. Chem. 2011, 50, 3354-3362; V. N. Kozhevnikov, K. Dahms, M. R. Bryce, J. Org. Chem. 2011, 76, 5143-51478; H. A. Al-Attar, G. C. Griffiths, T. N. Moore, M. Tavasli, M. A. Fox, M. R. Bryce, A. P. Monkman, Adv. Funct. Mater. 2011, 21, 2376-2382; Y. Zheng, A. S. Batsanov, R. M. Edkins, A. Beeby, M. R. Bryce, Inorg. Chem. 2012, 51, 290-297; M. Tavasli, T. N. Moore, Y. Zheng, M. R. Bryce, M. A. Fox, G. C. Griffiths, V. Jankus, H. A. Al-Attar, A. P. Monkman, J. Mater. Chem. 2012, DOI: c2jm15049b


/prod01/prodbucket01/media/durham-university/departments-/chemistry/71353.jpg)